Page 1149 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1149
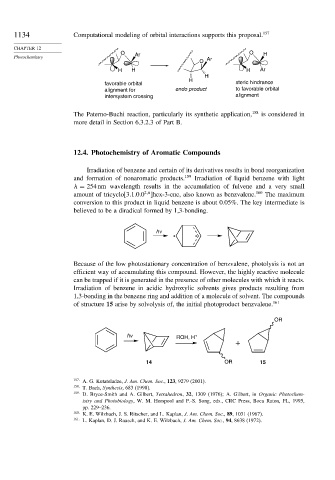
1134 Computational modeling of orbital interactions supports this proposal. 157
CHAPTER 12
O O H
Photochemistry Ar Ar
O
H H H Ar
H
H
favorable orbital steric hindrance
alignment for endo product to favorable orbital
intersystem crossing alignment
The Paterno-Buchi reaction, particularly its synthetic application, 158 is considered in
more detail in Section 6.3.2.3 of Part B.
12.4. Photochemistry of Aromatic Compounds
Irradiation of benzene and certain of its derivatives results in bond reorganization
and formation of nonaromatic products. 159 Irradiation of liquid benzene with light
= 254nm wavelength results in the accumulation of fulvene and a very small
2 6
amount of tricyclo[3.1.0.0 ]hex-3-ene, also known as benzvalene. 160 The maximum
conversion to this product in liquid benzene is about 0.05%. The key intermediate is
believed to be a diradical formed by 1,3-bonding.
h ν
Because of the low photostationary concentration of benzvalene, photolysis is not an
efficient way of accumulating this compound. However, the highly reactive molecule
can be trapped if it is generated in the presence of other molecules with which it reacts.
Irradiation of benzene in acidic hydroxylic solvents gives products resulting from
1,3-bonding in the benzene ring and addition of a molecule of solvent. The compounds
of structure 15 arise by solvolysis of, the initial photoproduct benzvalene. 161
OR
hv ROH, H +
+
14 OR 15
157
A. G. Kutateladze, J. Am. Chem. Soc., 123, 9279 (2001).
158
T. Bach, Synthesis, 683 (1998).
159 D. Bryce-Smith and A. Gilbert, Tetrahedron, 32, 1309 (1976); A. Gilbert, in Organic Photochem-
istry and Photobiology, W. M. Horspool and P.-S. Song, eds., CRC Press, Boca Raton, FL, 1995,
pp. 229–236.
160 K. E. Wilzbach, J. S. Ritscher, and L. Kaplan, J. Am. Chem. Soc., 89, 1031 (1967).
161
L. Kaplan, D. J. Rausch, and K. E. Wilzbach, J. Am. Chem. Soc., 94, 8638 (1972).