Page 1152 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1152
1137
TOPIC 12.1
1.549 Computational
Interpretation of Diene
and Polyene
1.576 Photochemistry
1.941 2.122
1.999
1.386
1.495 1.507
1.429
1.356 1.441
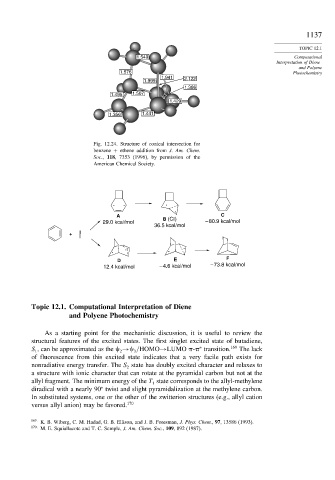
Fig. 12.24. Structure of conical intersection for
benzene + ethene addition from J. Am. Chem.
Soc., 118, 7353 (1996), by permission of the
American Chemical Society.
. . .
.
A C
29.0 kcal/mol B (CI) – 80.9 kcal/mol
36.5 kcal/mol
+
. .
. .
D E F
12.4 kcal/mol – 4.6 kcal/mol – 73.8 kcal/mol
Topic 12.1. Computational Interpretation of Diene
and Polyene Photochemistry
As a starting point for the mechanistic discussion, it is useful to review the
structural features of the excited states. The first singlet excited state of butadiene,
S , can be approximated as the → /HOMO→LUMO - transition. 169 The lack
∗
3
1
2
of fluorescence from this excited state indicates that a very facile path exists for
nonradiative energy transfer. The S state has doubly excited character and relaxes to
2
a structure with ionic character that can rotate at the pyramidal carbon but not at the
allyl fragment. The minimum energy of the T state corresponds to the allyl-methylene
1
diradical with a nearly 90 twist and slight pyramidalization at the methylene carbon.
In substituted systems, one or the other of the zwitterion structures (e.g., allyl cation
versus allyl anion) may be favored. 170
169 K. B. Wiberg, C. M. Hadad, G. B. Ellison, and J. B. Foresman, J. Phys. Chem., 97, 13586 (1993).
170
M. E. Squiallacote and T. C. Semple, J. Am. Chem. Soc., 109, 892 (1987).