Page 1151 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1151
1136
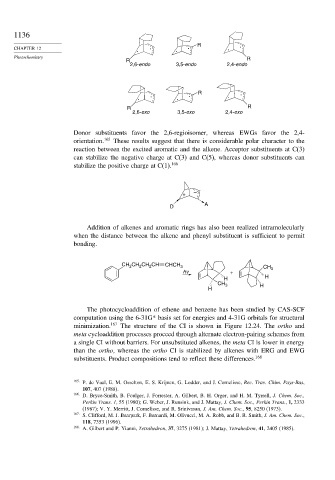
R .
CHAPTER 12 . . .
. .
Photochemistry
R R
2,6-endo 3,5-endo 2,4-endo
R .
. . .
. .
R R
2,6-exo 3,5-exo 2,4-exo
Donor substituents favor the 2,6-regioisomer, whereas EWGs favor the 2,4-
orientation. 165 These results suggest that there is considerable polar character to the
reaction between the excited aromatic and the alkene. Acceptor substituents at C(3)
can stabilize the negative charge at C(3) and C(5), whereas donor substituents can
stabilize the positive charge at C(1). 166
–
+
A
D
Addition of alkenes and aromatic rings has also been realized intramolecularly
when the distance between the alkene and phenyl substituent is sufficient to permit
bonding.
CH CH CH CH CHCH 3 CH
2
2
2
hv + 3
H H
CH 3 H
H
The photocycloaddition of ethene and benzene has been studied by CAS-SCF
computation using the 6-31G* basis set for energies and 4-31G orbitals for structural
minimization. 167 The structure of the CI is shown in Figure 12.24. The ortho and
meta cycloaddition processes proceed through alternate electron-pairing schemes from
a single CI without barriers. For unsubstituted alkenes, the meta CI is lower in energy
than the ortho, whereas the ortho CI is stabilized by alkenes with ERG and EWG
substituents. Product compositions tend to reflect these differences. 168
165
P. de Vaal, E. M. Osselton, E. S. Krijnen, G. Lodder, and J. Cornelisse, Rec. Trav. Chim. Pays-Bas,
107, 407 (1988).
166 D. Bryce-Smith, B. Foulger, J. Forrester, A. Gilbert, B. H. Orger, and H. M. Tyrrell, J. Chem. Soc.,
Perkin Trans. 1, 55 (1980); G. Weber, J. Runsink, and J. Mattay, J. Chem. Soc., Perkin Trans., 1, 2333
(1987); V. Y. Merritt, J. Cornelisse, and R. Srinivasan, J. Am. Chem. Soc., 95, 8250 (1973).
167 S. Clifford, M. J. Bearpark, F. Bernardi, M. Olivucci, M. A. Robb, and B. R. Smith, J. Am. Chem. Soc.,
118, 7353 (1996).
168
A. Gilbert and P. Yianni, Tetrahedron, 37, 3275 (1981); J. Mattay, Tetrahedron, 41, 2405 (1985).