Page 1148 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1148
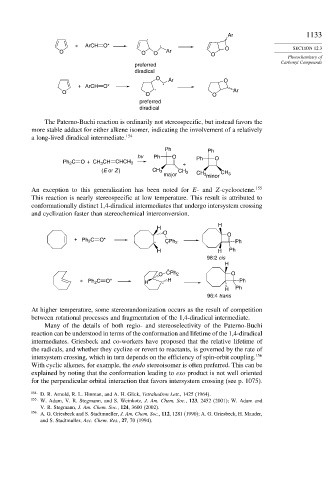
Ar 1133
+ ArCH O* O SECTION 12.3
O O O Ar O
Photochemistry of
Carbonyl Compounds
preferred
diradical
O Ar O
+ ArCH O*
O O O Ar
preferred
diradical
The Paterno-Buchi reaction is ordinarily not stereospecific, but instead favors the
more stable adduct for either alkene isomer, indicating the involvement of a relatively
a long-lived diradical intermediate. 154
Ph Ph
hv Ph O Ph O
Ph 2 C O + CH CH CHCH 3 +
3
(E or Z ) CH 3 CH 3
major CH 3 minor CH 3
An exception to this generalization has been noted for E- and Z-cyclooctene. 155
This reaction is nearly stereospecific at low temperature. This result is attributed to
conformationally distinct 1,4-diradical intermediates that undergo intersystem crossing
and cyclization faster than stereochemical interconversion.
H
H
O O
+ Ph 2 C O*
CPh 2 Ph
H H Ph
98:2 cis
H
O CPh 2 O
+ Ph 2 C O* H H Ph
H Ph
96:4 trans
At higher temperature, some stereorandomization occurs as the result of competition
between rotational processes and fragmentation of the 1,4-diradical intermediate.
Many of the details of both regio- and stereoselectivity of the Paterno-Buchi
reaction can be understood in terms of the conformation and lifetime of the 1,4-diradical
intermediates. Griesbeck and co-workers have proposed that the relative lifetime of
the radicals, and whether they cyclize or revert to reactants, is governed by the rate of
intersystem crossing, which in turn depends on the efficiency of spin-orbit coupling. 156
With cyclic alkenes, for example, the endo stereoisomer is often preferred. This can be
explained by noting that the conformation leading to exo product is not well oriented
for the perpendicular orbital interaction that favors intersystem crossing (see p. 1075).
154 D. R. Arnold, R. L. Hinman, and A. H. Glick, Tetrahedron Lett., 1425 (1964).
155 W. Adam, V. R. Stegmann, and S. Weinkotz, J. Am. Chem. Soc., 123, 2452 (2001); W. Adam and
V. R. Stegmann, J. Am. Chem. Soc., 124, 3600 (2002).
156
A. G. Griesbeck and S. Stadtmueller, J. Am. Chem. Soc., 112, 1281 (1990); A. G. Griesbeck, H. Mauder,
and S. Stadtmuller, Acc. Chem. Res., 27, 70 (1994).