Page 1150 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1150
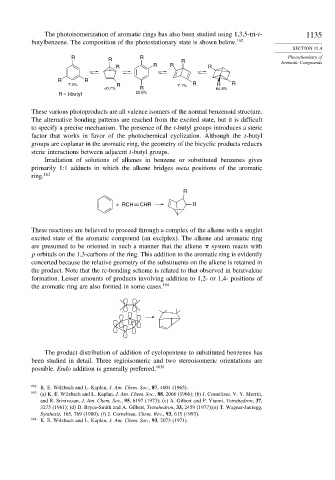
The photoisomerization of aromatic rings has also been studied using 1,3,5-tri-t- 1135
butylbenzene. The composition of the photostationary state is shown below. 162
SECTION 12.4
Photochemistry of
R R R R Aromatic Compounds
R R R R
R R
7.3% R 7.1% R R R
<0.7% R 64.8%
R = t-butyl 20.6%
These various photoproducts are all valence isomers of the normal benzenoid structure.
The alternative bonding patterns are reached from the excited state, but it is difficult
to specify a precise mechanism. The presence of the t-butyl groups introduces a steric
factor that works in favor of the photochemical cyclization. Although the t-butyl
groups are coplanar in the aromatic ring, the geometry of the bicyclic products reduces
steric interactions between adjacent t-butyl groups.
Irradiation of solutions of alkenes in benzene or substituted benzenes gives
primarily 1:1 adducts in which the alkene bridges meta positions of the aromatic
ring. 163
R
+ RCH CHR R
These reactions are believed to proceed through a complex of the alkene with a singlet
excited state of the aromatic compound (an exciplex). The alkene and aromatic ring
are presumed to be oriented in such a manner that the alkene system reacts with
p orbitals on the 1,3-carbons of the ring. This addition to the aromatic ring is evidently
concerted because the relative geometry of the substituents on the alkene is retained in
the product. Note that the re-bonding scheme is related to that observed in benzvalene
formation. Lesser amounts of products involving addition to 1,2- or 1,4- positions of
the aromatic ring are also formed in some cases. 164
The product distribution of addition of cyclopentene to substituted benzenes has
been studied in detail. Three regioisomeric and two stereoisomeric orientations are
possible. Endo addition is generally preferred. 163f
162 K. E. Wilzbach and L. Kaplan, J. Am. Chem. Soc., 87, 4004 (1965).
163 (a) K. E. Wilzbach and L. Kaplan, J. Am. Chem. Soc., 88, 2066 (1966); (b) J. Cornelisse, V. Y. Merritt,
and R. Srinivasan, J. Am. Chem. Soc., 95, 6197 (1973); (c) A. Gilbert and P. Yianni, Tetrahedron, 37,
3275 (1981); (d) D. Bryce-Smith and A. Gilbert, Tetrahedron, 33, 2459 (1977);(e) T. Wagner-Jauregg,
Synthesis, 165, 769 (1980); (f) J. Cornelisse, Chem. Rev., 93, 615 (1993).
164
K. E. Wilzbach and L. Kaplan, J. Am. Chem. Soc., 93, 2073 (1971).