Page 1153 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1153
1138 As computational methods for describing excited states have been refined,
additional understanding of the structures has developed. Relatively early computa-
CHAPTER 12 tional studies provided some indication of the geometries associated with the butadiene
Photochemistry excited states. 171 The ground state has a maximum at a twist of 90 about the C(1)−C(2)
bond. This structure, which can be approximately described as a singlet methylene-allyl
diradical, is found at about 2.3 eV and is more stable than a structure with 90 twist
at both terminal groups (3.1 eV). There is no major pyramidalization of the methylene
groups in this second structure. The spectroscopic (Franck-Condon) T state is about
1
3.5 eV above S .A local planar minimum is found to have a shortened C(2)−C(3)
0
bond and lengthened C(1)−C(2) and C(3)−C(4) bonds The energy of this structure is
about 2.7 eV. The C(2)−C(3) bond distance is 1.36 Å, so the bond has considerable
double-bond character. This local minimum can be represented as a 1,4-but-2-enyl
diradical. The global minimum on the T surface is a twisted triplet allyl-methylene
1
diradical that is at about 2.3 eV. This structure is very similar in geometry to the
singlet diradical on the ground state surface.
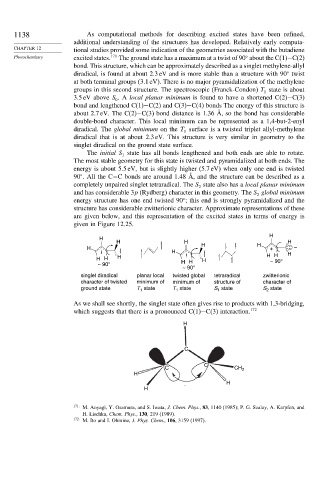
The initial S state has all bonds lengthened and both ends are able to rotate.
1
The most stable geometry for this state is twisted and pyramidalized at both ends. The
energy is about 5.5 eV, but is slightly higher (5.7 eV) when only one end is twisted
90 . All the C−C bonds are around 1.48 Å, and the structure can be described as a
completely unpaired singlet tetraradical. The S state also has a local planar minimum
2
and has considerable 3p (Rydberg) character in this geometry. The S global minimum
2
energy structure has one end twisted 90 ; this end is strongly pyramidalized and the
structure has considerable zwitterionic character. Approximate representations of these
are given below, and this representation of the excited states in terms of energy is
given in Figure 12.25.
H
H
H H H
H H H + : –
H H
H
H H H H H H ~ 90°
~ 90° H
~ 90°
singlet diradical planar local twisted global tetraradical zwitterionic
character of twisted minimum of minimum of structure of character of
ground state T state T state S state S state
1
1
1
2
As we shall see shortly, the singlet state often gives rise to products with 1,3-bridging,
which suggests that there is a pronounced C(1)−C(3) interaction. 172
H
C
C
C CH 2
H
H
H
171 M. Aoyagi, Y. Osamura, and S. Iwata, J. Chem. Phys., 83, 1140 (1985); P. G. Szalay, A. Karpfen, and
H. Lischka, Chem. Phys., 130, 219 (1989).
172
M. Ito and I. Ohmine, J. Phys. Chem., 106, 3159 (1997).