Page 1158 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1158
In this structure C(1) is actually a bit closer to C(5) than to C(6). The C(2,3,4) 1143
fragment resembles an allyl radical. There are recoupling patterns that can lead either
to cyclohexadiene or to Z-1,3,5-hexatriene. TOPIC 12.1
Computational
1-6/4-5 1-2/5-6 Interpretation of Diene
coupling 3 . 4 coupling and Polyene
2 . 5 Photochemistry
. .
1 6
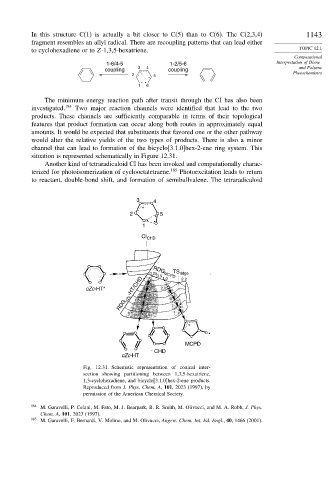
The minimum energy reaction path after transit through the CI has also been
investigated. 184 Two major reaction channels were identified that lead to the two
products. These channels are sufficiently comparable in terms of their topological
features that product formation can occur along both routes in approximately equal
amounts. It would be expected that substituents that favored one or the other pathway
would alter the relative yields of the two types of products. There is also a minor
channel that can lead to formation of the bicyclo[3.1.0]hex-2-ene ring system. This
situation is represented schematically in Figure 12.31.
Another kind of tetraradicaloid CI has been invoked and computationally charac-
terized for photoisomerization of cyclooctatetraene. 185 Photoexcitation leads to return
to reactant, double-bond shift, and formation of semibullvalene. The tetraradicaloid
3 4
2 5
1
CI CHD
TS
0.25 0.5 1.0 ridge
RDG MCPD
RDG cZc -HT/CHD
2.2
cZc-HT* 2.0
MCPD
CHD
cZc-HT
Fig. 12.31. Schematic representation of conical inter-
section showing partitioning between 1,3,5-hexatriene,
1,3-cyclohexadiene, and bicyclo[3.1.0]hex-2-ene products.
Reproduced from J. Phys. Chem. A, 101, 2023 (1997), by
permission of the American Chemical Society.
184 M. Garavelli, P. Celani, M. Fato, M. J. Bearpark, B. R. Smith, M. Olivucci, and M. A. Robb, J. Phys.
Chem. A, 101, 2023 (1997).
185
M. Garavelli, F. Bernardi, V. Molino, and M. Olivucci, Angew. Chem. Int. Ed. Engl., 40, 1466 (2001).