Page 1157 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1157
1142
CHAPTER 12
S 2
Photochemistry
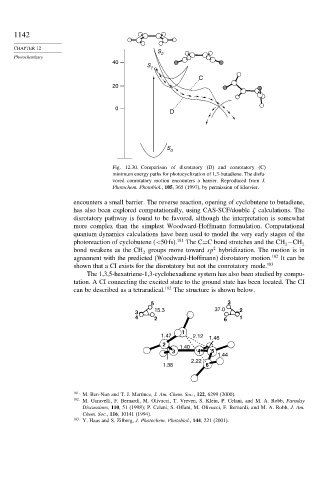
40
S
1
C
20
0
D
S
0
Fig. 12.30. Comparison of disrotatory (D) and conrotatory (C)
minimum energy paths for photocyclization of 1,3-butadiene. The disfa-
vored conrotatory motion encounters a barrier. Reproduced from J.
Photochem. Photobiol., 105, 365 (1997), by permission of Elsevier.
encounters a small barrier. The reverse reaction, opening of cyclobutene to butadiene,
has also been explored computationally, using CAS-SCF/double calculations. The
disrotatory pathway is found to be favored, although the interpretation is somewhat
more complex than the simplest Woodward-Hoffmann formulation. Computational
quantum dynamics calculations have been used to model the very early stages of the
photoreaction of cyclobutene (<50fs). 181 The C=C bond stretches and the CH −CH 2
2
2
bond weakens as the CH groups move toward sp hybridization. The motion is in
2
agreement with the predicted (Woodward-Hoffmann) disrotatory motion. 182 It can be
shown that a CI exists for the disrotatory but not the conrotatory mode. 183
The 1,3,5-hexatriene-1,3-cyclohexadiene system has also been studied by compu-
tation. A CI connecting the excited state to the ground state has been located. The CI
can be described as a tetraradical. 182 The structure is shown below.
5 3
15.3 37.0 2
3
4 2 6 1
1
1.47 2.12 1.46
2
1.40
3 4 5
1.44
2.22
1.38 6
181
M. Ben-Nun and T. J. Martinez, J. Am. Chem. Soc., 122, 6299 (2000).
182 M. Garavelli, F. Bernardi, M. Olivucci, T. Vreven, S. Klein, P. Celani, and M. A. Robb, Faraday
Discussions, 110, 51 (1998); P. Celani, S. Offani, M. Olivucci, F. Bernardi, and M. A. Robb, J. Am.
Chem. Soc., 116, 10141 (1994).
183
Y. Haas and S. Zilberg, J. Photochem. Photobiol., 144, 221 (2001).