Page 1146 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1146
experiments have indicated that the reaction proceeds through a triplet excited state. 1131
A scheme that delineates the bonding changes is outlined below.
SECTION 12.3
O O O – O O
Photochemistry of
+ + Ph Carbonyl Compounds
Ph
–
C Ph 2
Ph Ph Ph Ph D Ph Ph 10
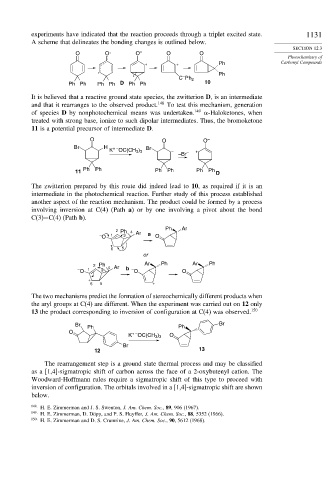
It is believed that a reactive ground state species, the zwitterion D, is an intermediate
and that it rearranges to the observed product. 148 To test this mechanism, generation
of species D by nonphotochemical means was undertaken. 149 -Haloketones, when
treated with strong base, ionize to such dipolar intermediates. Thus, the bromoketone
11 is a potential precursor of intermediate D.
O O O –
Br H + – Br
K OC(CH 3 ) 3 – – +
–Br
Ph Ph
11 Ph Ph Ph Ph D
The zwitterion prepared by this route did indeed lead to 10, as required if it is an
intermediate in the photochemical reaction. Further study of this process established
another aspect of the reaction mechanism. The product could be formed by a process
involving inversion at C(4) (Path a) or by one involving a pivot about the bond
C(3)−C(4) (Path b).
Ph Ar
2 Ph 4 Ar
– O 1 3 a O
+
6 5
or
2 Ph Ar Ph Ar Ph
– 1 3 4 Ar b –
O O O
+
6 5 +
The two mechanisms predict the formation of stereochemically different products when
the aryl groups at C(4) are different. When the experiment was carried out on 12 only
13 the product corresponding to inversion of configuration at C(4) was observed. 150
Br Ph Ph Br
O + –
K OC(CH 3 ) 3 O
Br
12 13
The rearrangement step is a ground state thermal process and may be classified
as a [1,4]-sigmatropic shift of carbon across the face of a 2-oxybutenyl cation. The
Woodward-Hoffmann rules require a sigmatropic shift of this type to proceed with
inversion of configuration. The orbitals involved in a [1,4]-sigmatropic shift are shown
below.
148 H. E. Zimmerman and J. S. Swenton, J. Am. Chem. Soc., 89, 906 (1967).
149 H. E. Zimmerman, D. Döpp, and P. S. Huyffer, J. Am. Chem. Soc., 88, 5352 (1966).
150
H. E. Zimmerman and D. S. Crumrine, J. Am. Chem. Soc., 90, 5612 (1968).