Page 140 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 140
120 ligand atoms. When the substituents at the two carbons are nonidentical, two struc-
turally distinct molecules exist.
CHAPTER 2
Stereochemistry,
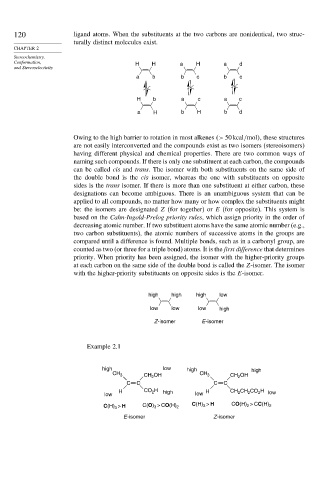
Conformation, H H a H a d
and Stereoselectivity
a b b c b c
H b a c a c
a H b H b d
Owing to the high barrier to rotation in most alkenes > 50kcal/mol , these structures
are not easily interconverted and the compounds exist as two isomers (stereoisomers)
having different physical and chemical properties. There are two common ways of
naming such compounds. If there is only one substituent at each carbon, the compounds
can be called cis and trans. The isomer with both substituents on the same side of
the double bond is the cis isomer, whereas the one with substituents on opposite
sides is the trans isomer. If there is more than one substituent at either carbon, these
designations can become ambiguous. There is an unambiguous system that can be
applied to all compounds, no matter how many or how complex the substituents might
be: the isomers are designated Z (for together) or E (for opposite). This system is
based on the Cahn-Ingold-Prelog priority rules, which assign priority in the order of
decreasing atomic number. If two substituent atoms have the same atomic number (e.g.,
two carbon substituents), the atomic numbers of successive atoms in the groups are
compared until a difference is found. Multiple bonds, such as in a carbonyl group, are
counted as two (or three for a triple bond) atoms. It is the first difference that determines
priority. When priority has been assigned, the isomer with the higher-priority groups
at each carbon on the same side of the double bond is called the Z-isomer. The isomer
with the higher-priority substituents on opposite sides is the E-isomer.
high high high low
low low low high
Z -isomer E -isomer
Example 2.1
high low high high
CH 3 CH 2 OH CH 3 CH 2 OH
C C C C
H CO H high H CH 2 CH CO H low
2
low low 2 2
3
2
C(H) > H C(O) 3 > CO(H) 2 C(H) > H CO(H) > CC(H) 2
3
E -isomer Z -isomer