Page 143 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 143
The two nonsuperimposable mirror image molecules are called an enantiomeric 123
pair and each is the enantiomer of the other. The separated enantiomers have identical
properties with respect to achiral environments. They have the same solubility, SECTION 2.1
physical, and spectroscopic properties and the same chemical reactivity toward Configuration
achiral reagents. However, they have different properties in chiral environments. The
enantiomers react at different rates toward chiral reagents and respond differently to
chiral catalysts. Usually enantiomers cause differing physiological responses, since
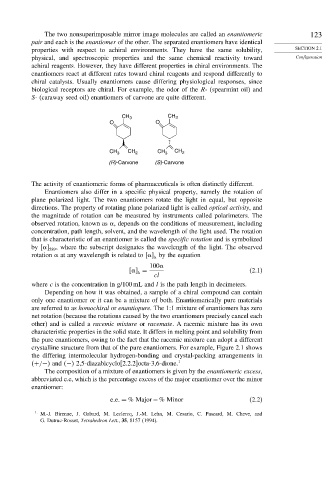
biological receptors are chiral. For example, the odor of the R- (spearmint oil) and
S- (caraway seed oil) enantiomers of carvone are quite different.
CH 3 CH 3
O O
CH 3 CH 2 CH 3 CH 2
(R)-Carvone (S)-Carvone
The activity of enantiomeric forms of pharmaceuticals is often distinctly different.
Enantiomers also differ in a specific physical property, namely the rotation of
plane polarized light. The two enantiomers rotate the light in equal, but opposite
directions. The property of rotating plane polarized light is called optical activity, and
the magnitude of rotation can be measured by instruments called polarimeters. The
observed rotation, known as , depends on the conditions of measurement, including
concentration, path length, solvent, and the wavelength of the light used. The rotation
that is characteristic of an enantiomer is called the specific rotation and is symbolized
by 589 , where the subscript designates the wavelength of the light. The observed
rotation at any wavelength is related to by the equation
100
= (2.1)
cl
where c is the concentration in g/100 mL and l is the path length in decimeters.
Depending on how it was obtained, a sample of a chiral compound can contain
only one enantiomer or it can be a mixture of both. Enantiomerically pure materials
are referred to as homochiral or enantiopure. The 1:1 mixture of enantiomers has zero
net rotation (because the rotations caused by the two enantiomers precisely cancel each
other) and is called a racemic mixture or racemate. A racemic mixture has its own
characteristic properties in the solid state. It differs in melting point and solubility from
the pure enantiomers, owing to the fact that the racemic mixture can adopt a different
crystalline structure from that of the pure enantiomers. For example, Figure 2.1 shows
the differing intermolecular hydrogen-bonding and crystal-packing arrangements in
+/− and − 2,5-diazabicyclo[2.2.2]octa-3,6-dione. 1
The composition of a mixture of enantiomers is given by the enantiomeric excess,
abbreviated e.e, which is the percentage excess of the major enantiomer over the minor
enantiomer:
e e = % Major −% Minor (2.2)
1
M.-J. Birenne, J. Gabard, M. Leclercq, J.-M. Lehn, M. Cesario, C. Pascard, M. Cheve, and
G. Dutruc-Rosset, Tetrahedron Lett., 35, 8157 (1994).