Page 146 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 146
126
CHAPTER 2
Stereochemistry,
Conformation,
and Stereoselectivity
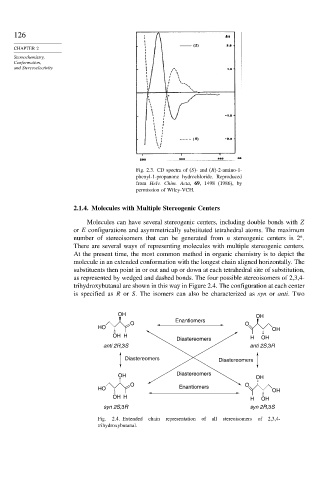
Fig. 2.3. CD spectra of (S)- and (R)-2-amino-1-
phenyl-1-propanone hydrochloride. Reproduced
from Helv. Chim. Acta, 69, 1498 (1986), by
permission of Wiley-VCH.
2.1.4. Molecules with Multiple Stereogenic Centers
Molecules can have several stereogenic centers, including double bonds with Z
or E configurations and asymmetrically substituted tetrahedral atoms. The maximum
n
number of stereoisomers that can be generated from n stereogenic centers is 2 .
There are several ways of representing molecules with multiple stereogenic centers.
At the present time, the most common method in organic chemistry is to depict the
molecule in an extended conformation with the longest chain aligned horizontally. The
substituents then point in or out and up or down at each tetrahedral site of substitution,
as represented by wedged and dashed bonds. The four possible stereoisomers of 2,3,4-
trihydroxybutanal are shown in this way in Figure 2.4. The configuration at each center
is specified as R or S. The isomers can also be characterized as syn or anti. Two
OH OH
Enantiomers
O O
HO OH
OH H
Diastereomers H OH
anti 2R,3S anti 2S,3R
Diastereomers Diastereomers
OH Diastereomers OH
O O
HO Enantiomers OH
OH H H OH
syn 2S,3R syn 2R,3S
Fig. 2.4. Extended chain representation of all stereoisomers of 2,3,4-
trihydroxybutanal.