Page 150 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 150
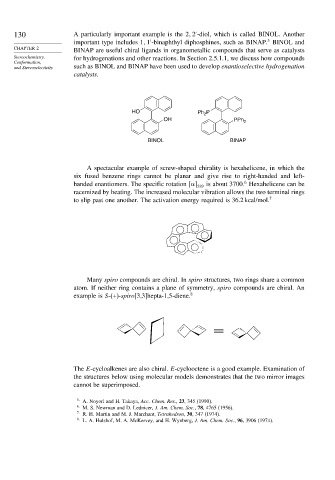
130 A particularly important example is the 2 2 -diol, which is called BINOL. Another
5
important type includes 1 1 -binaphthyl diphosphines, such as BINAP. BINOL and
CHAPTER 2
BINAP are useful chiral ligands in organometallic compounds that serve as catalysts
Stereochemistry, for hydrogenations and other reactions. In Section 2.5.1.1, we discuss how compounds
Conformation,
and Stereoselectivity such as BINOL and BINAP have been used to develop enantioselective hydrogenation
catalysts.
HO Ph P
2
OH PPh 2
BINOL BINAP
A spectacular example of screw-shaped chirality is hexahelicene, in which the
six fused benzene rings cannot be planar and give rise to right-handed and left-
6
handed enantiomers. The specific rotation 589 is about 3700. Hexahelicene can be
racemized by heating. The increased molecular vibration allows the two terminal rings
to slip past one another. The activation energy required is 36.2 kcal/mol. 7
Many spiro compounds are chiral. In spiro structures, two rings share a common
atom. If neither ring contains a plane of symmetry, spiro compounds are chiral. An
example is S-(+)-spiro[3,3]hepta-1,5-diene. 8
The E-cycloalkenes are also chiral. E-cyclooctene is a good example. Examination of
the structures below using molecular models demonstrates that the two mirror images
cannot be superimposed.
5
A. Noyori and H. Takaya, Acc. Chem. Res., 23, 345 (1990).
6
M. S. Newman and D. Lednicer, J. Am. Chem. Soc., 78, 4765 (1956).
7 R. H. Martin and M. J. Marchant, Tetrahedron, 30, 347 (1974).
8
L. A. Hulshof, M. A. McKervey, and H. Wynberg, J. Am. Chem. Soc., 96, 3906 (1974).