Page 152 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 152
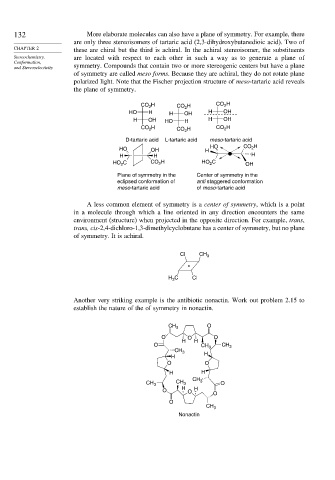
132 More elaborate molecules can also have a plane of symmetry. For example, there
are only three stereoisomers of tartaric acid (2,3-dihydroxybutanedioic acid). Two of
CHAPTER 2 these are chiral but the third is achiral. In the achiral stereoisomer, the substituents
Stereochemistry, are located with respect to each other in such a way as to generate a plane of
Conformation,
and Stereoselectivity symmetry. Compounds that contain two or more stereogenic centers but have a plane
of symmetry are called meso forms. Because they are achiral, they do not rotate plane
polarized light. Note that the Fischer projection structure of meso-tartaric acid reveals
the plane of symmetry.
CO H CO H CO 2 H
2
2
HO H H OH H OH
H OH HO H H OH
CO H CO H CO H
2
2
2
D-tartaric acid L-tartaric acid meso-tartaric acid
2
HO OH H HO CO H
H H H
HO C CO H HO 2 C OH
2
2
Plane of symmetry in the Center of symmetry in the
eclipsed conformation of anti staggered conformation
meso-tartaric acid of meso-tartaric acid
A less common element of symmetry is a center of symmetry, which is a point
in a molecule through which a line oriented in any direction encounters the same
environment (structure) when projected in the opposite direction. For example, trans,
trans, cis-2,4-dichloro-1,3-dimethylcyclobutane has a center of symmetry, but no plane
of symmetry. It is achiral.
Cl CH 3
H C Cl
3
Another very striking example is the antibiotic nonactin. Work out problem 2.15 to
establish the nature of the of symmetry in nonactin.
CH 3 O
O O O
H H
O CH 3 CH 3
CH 3
H H
O O
H H
CH 3 CH 3 CH 3 O
O H O H O
O
CH 3
Nonactin