Page 157 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 157
Scheme 2.3. Resolution of 3-Methyl-2-Phenylbutanoic 137
Acid a
SECTION 2.1
Ph Ph
H H Configuration
CO 2 H HO C
2
(CH ) CH CH(CH )
3 2
3 2
racemic mixture, 461 g CH 3
Ph
form salt with NH 2
H
R-(+)
mixture of 353 g of diastereomeric ammonium
carboxylate salts recrystallized from ethanol-water
recrystallized salt from
product filtrate
R,R salt, 272 g mp 198 – 200° C enriched in S,R- salt
acidify acidify
R-(–) acid 153 g partially resolved
mp 50.5 – 51.5° C S-acid, 261 g, [ α] + 36
[α] – 62.4
* a. C. Aaron, D. Dull, J. L. Schmiegel, D. Jaeger, Y. Ohahi, and
H. S. Mosher, J. Org. Chem., 32, 2797 (1967).
Another means of resolution is to use a chiral material in a physical separation.
Currently, many resolutions are done using medium- or high-pressure chromatography
with chiral column-packing materials. Resolution by chromatography depends upon
differential adsorption of the enantiomers by the chiral stationary phase. Differential
adsorption occurs because of the different “fit” of the two enantiomers to the chiral
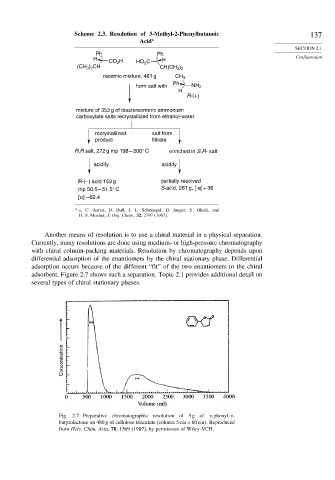
adsorbent. Figure 2.7 shows such a separation. Topic 2.1 provides additional detail on
several types of chiral stationary phases.
Fig. 2.7. Preparative chromatographic resolution of 5 g of
-phenyl-
-
butyrolactone on 480 g of cellulose triacetate (column 5cm ×60cm). Reproduced
from Helv. Chim. Acta, 70, 1569 (1987), by permission of Wiley-VCH.