Page 162 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 162
142 2.2. Conformation
CHAPTER 2
The structural aspects of stereochemistry discussed in the previous section are
Stereochemistry,
Conformation, the consequences of configuration, the geometric arrangement fixed by the chemical
and Stereoselectivity bonds within the molecule. Now, we want to look at another level of molecular
structure, conformation. Conformations are the different shapes that a molecule can
attain without breaking any covalent bonds. They differ from one another as the result
of rotation at one or more single bond. The energy barrier for rotation of carbon-carbon
single bonds is normally small, less than 5 kcal/mol, but processes that involve several
coordinated rotations can have higher energy requirements. Conformational analysis
is the process of relating conformation to the properties and reactivity of molecules.
2.2.1. Conformation of Acyclic Compounds
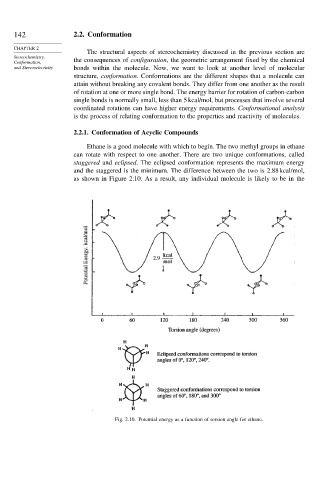
Ethane is a good molecule with which to begin. The two methyl groups in ethane
can rotate with respect to one another. There are two unique conformations, called
staggered and eclipsed. The eclipsed conformation represents the maximum energy
and the staggered is the minimum. The difference between the two is 2.88 kcal/mol,
as shown in Figure 2.10. As a result, any individual molecule is likely to be in the
Fig. 2.10. Potential energy as a function of torsion angle for ethane.