Page 167 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 167
With more highly substituted terminal alkenes, additional conformations are 147
available, as indicated for 1-butene.
SECTION 2.2
Conformation
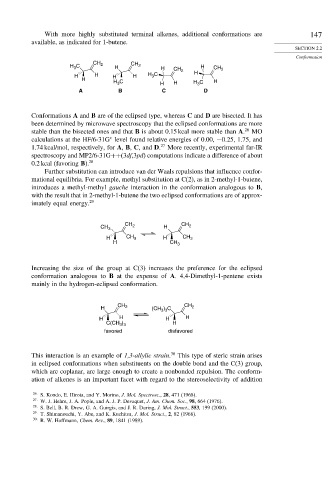
CH
H C 2 H CH 2 H CH 2 H CH 2
3
H H H H H C H
3
H H C H H H C H
3
3
A B C D
Conformations A and B are of the eclipsed type, whereas C and D are bisected. It has
been determined by microwave spectroscopy that the eclipsed conformations are more
stable than the bisected ones and that B is about 0.15 kcal more stable than A. 26 MO
calculations at the HF/6-31G level found relative energies of 0.00, −0 25, 1.75, and
∗
1.74 kcal/mol, respectively, for A, B, C, and D. 27 More recently, experimental far-IR
spectroscopy and MP2/6-31G++(3df,3pd) computations indicate a difference of about
0.2 kcal (favoring B). 28
Further substitution can introduce van der Waals repulsions that influence confor-
mational equilibria. For example, methyl substitution at C(2), as in 2-methyl-1-butene,
introduces a methyl-methyl gauche interaction in the conformation analogous to B,
with the result that in 2-methyl-1-butene the two eclipsed conformations are of approx-
imately equal energy. 29
CH CH
CH 3 2 H 2
H CH 3 H CH 3
H CH 3
Increasing the size of the group at C(3) increases the preference for the eclipsed
conformation analogous to B at the expense of A. 4,4-Dimethyl-1-pentene exists
mainly in the hydrogen-eclipsed conformation.
CH
H CH 2 (CH ) C 2
3 3
H H H H
C(CH ) H
3 3
favored disfavored
This interaction is an example of 1,3-allylic strain. 30 This type of steric strain arises
in eclipsed conformations when substituents on the double bond and the C(3) group,
which are coplanar, are large enough to create a nonbonded repulsion. The conform-
ation of alkenes is an important facet with regard to the stereoselectivity of addition
26 S. Kondo, E. Hirota, and Y. Morino, J. Mol. Spectrosc., 28, 471 (1968).
27
W. J. Hehre, J. A. Pople, and A. J. P. Devaquet, J. Am. Chem. Soc., 98, 664 (1976).
28
S. Bell, B. R. Drew, G. A. Guirgis, and J. R. During, J. Mol. Struct., 553, 199 (2000).
29 T. Shimanouchi, Y. Abe, and K. Kuchitsu, J. Mol. Struct., 2, 82 (1968).
30
R. W. Hoffmann, Chem. Rev., 89, 1841 (1989).