Page 164 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 164
144
CHAPTER 2
Stereochemistry,
Conformation,
and Stereoselectivity
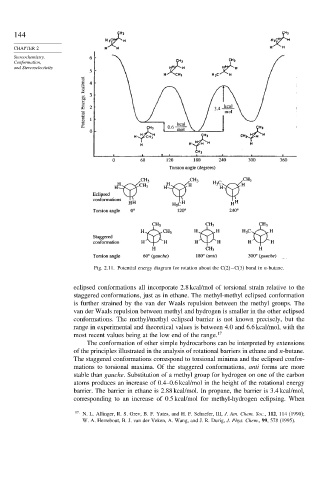
Fig. 2.11. Potential energy diagram for rotation about the C(2)−C(3) bond in n-butane.
eclipsed conformations all incorporate 2.8 kcal/mol of torsional strain relative to the
staggered conformations, just as in ethane. The methyl-methyl eclipsed conformation
is further strained by the van der Waals repulsion between the methyl groups. The
van der Waals repulsion between methyl and hydrogen is smaller in the other eclipsed
conformations. The methyl/methyl eclipsed barrier is not known precisely, but the
range in experimental and theoretical values is between 4.0 and 6.6 kcal/mol, with the
most recent values being at the low end of the range. 17
The conformation of other simple hydrocarbons can be interpreted by extensions
of the principles illustrated in the analysis of rotational barriers in ethane and n-butane.
The staggered conformations correspond to torsional minima and the eclipsed confor-
mations to torsional maxima. Of the staggered conformations, anti forms are more
stable than gauche. Substitution of a methyl group for hydrogen on one of the carbon
atoms produces an increase of 0.4–0.6 kcal/mol in the height of the rotational energy
barrier. The barrier in ethane is 2.88 kcal/mol. In propane, the barrier is 3.4 kcal/mol,
corresponding to an increase of 0.5 kcal/mol for methyl-hydrogen eclipsing. When
17
N. L. Allinger, R. S. Grev, B. F. Yates, and H. F. Schaefer, III, J. Am. Chem. Soc., 112, 114 (1990);
W. A. Herrebout, B. J. van der Veken, A. Wang, and J. R. Durig, J. Phys. Chem., 99, 578 (1995).