Page 159 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 159
Of course, the high conversion required for high enantiomeric purity when the relative 139
reactivity difference is low has a serious drawback. The yield of the unreacted substrate
is low if the overall conversion is high. Relative reactivity differences of < 10 can SECTION 2.1
achieve high enantiomeric purity only at the expense of low yield. Configuration
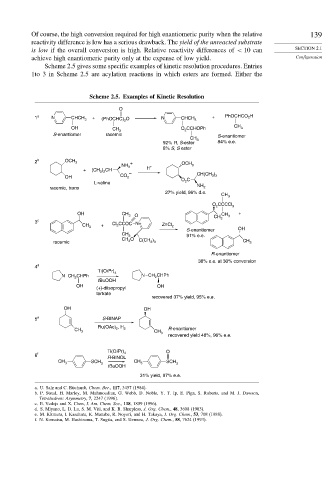
Scheme 2.5 gives some specific examples of kinetic resolution procedures. Entries
1to 3 in Scheme 2.5 are acylation reactions in which esters are formed. Either the
Scheme 2.5. Examples of Kinetic Resolution
O
1 a N CHCH 3 + (PhOCHC) 2 O N CHCH 3 + PhOCHCO 2 H
OH CH O CCHOPh CH 3
2
S-enantiomer racemic 3 S-enantiomer
CH
92% R, S-ester 3 84% e.e.
8% S, S ester
2 b OCH 3 + OCH
NH 3 + 3
+ (CH ) CH H
3 2
OH CO 2 – O C CH(CH 3 ) 2
L-valine 2
racemic, trans NH 2
27% yield, 96% d.e.
CH
3
O CCCCl 3
2
OH CH 3 O CH CH 3 +
3 c 3
CH 3 + Cl CCOC N+ ZnCl 2
3
S-enantiomer OH
91% e.e.
CH 3
CH 3 O C(CH )
racemic 3 3 CH 3
R-enantiomer
38% e.e. at 30% conversion
4 d
Ti(Oi Pr) 4
N CH CHPh N CH CHPh
2
2
t BuOOH
OH OH
(+)-diisopropyl
tartrate
recovered 37% yield, 95% e.e.
OH OH
5 e S-BINAP
Ru(OAc) , H R-enantiomer
CH 3 2 2 CH
3
recovered yield 48%, 96% e.e.
Ti(Oi Pr) 4 O
6 f
R-BINOL
CH SCH CH SCH
3 3 3 3
t BuOOH
31% yield, 97% e.e.
a. U. Salz and C. Rüchardt, Chem. Ber., 117, 3457 (1984).
b. P. Stead, H. Marley, M. Mahmoudian, G. Webb, D. Noble, Y. T. Ip, E. Piga, S. Roberts, and M. J. Dawson,
Tetrahedron: Asymmetry, 7, 2247 (1996).
c. E. Vedejs and X. Chen, J. Am. Chem. Soc., 118, 1809 (1996).
d. S. Miyano, L. D. Lu, S. M. Viti, and K. B. Sharpless, J. Org. Chem., 48, 3608 (1983).
e. M. Kitmura, I. Kasahara, K. Manabe, R. Noyori, and H. Takaya, J. Org. Chem., 53, 708 (1988).
f. N. Komatsu, M. Hashizuma, T. Sugita, and S. Uemura, J. Org. Chem., 58, 7624 (1993).