Page 158 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 158
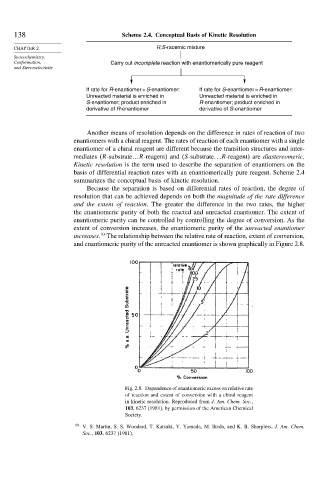
138 Scheme 2.4. Conceptual Basis of Kinetic Resolution
CHAPTER 2 R,S-racemic mixture
Stereochemistry,
Conformation, Carry out incomplete reaction with enantiomerically pure reagent
and Stereoselectivity
If rate for R-enantiomer > S-enantiomer: If rate for S-enantiomer > R-enantiomer:
Unreacted material is enriched in Unreacted material is enriched in
S-enantiomer; product enriched in R-enantiomer; product enriched in
derivative of R-enantiomer derivative of S-enantiomer
Another means of resolution depends on the difference in rates of reaction of two
enantiomers with a chiral reagent. The rates of reaction of each enantiomer with a single
enantiomer of a chiral reagent are different because the transition structures and inter-
mediates (R-substrate…R-reagent) and (S-substrate R-reagent) are diastereomeric.
Kinetic resolution is the term used to describe the separation of enantiomers on the
basis of differential reaction rates with an enantiomerically pure reagent. Scheme 2.4
summarizes the conceptual basis of kinetic resolution.
Because the separation is based on differential rates of reaction, the degree of
resolution that can be achieved depends on both the magnitude of the rate difference
and the extent of reaction. The greater the difference in the two rates, the higher
the enantiomeric purity of both the reacted and unreacted enantiomer. The extent of
enantiomeric purity can be controlled by controlling the degree of conversion. As the
extent of conversion increases, the enantiomeric purity of the unreacted enantiomer
10
increases. The relationship between the relative rate of reaction, extent of conversion,
and enantiomeric purity of the unreacted enantiomer is shown graphically in Figure 2.8.
Fig. 2.8. Dependence of enantiomeric excess on relative rate
of reaction and extent of conversion with a chiral reagent
in kinetic resolution. Reproduced from J. Am. Chem. Soc.,
103, 6237 (1981), by permission of the American Chemical
Society.
10
V. S. Martin, S. S. Woodard, T. Katsuki, Y. Yamada, M. Ikeda, and K. B. Sharpless, J. Am. Chem.
Soc., 103, 6237 (1981).