Page 153 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 153
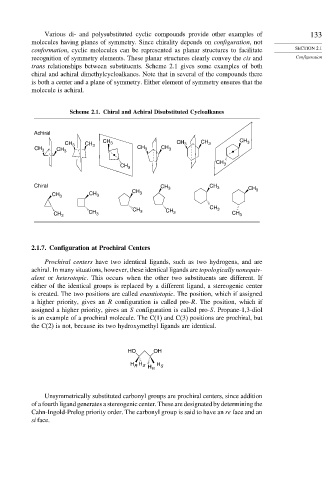
Various di- and polysubstituted cyclic compounds provide other examples of 133
molecules having planes of symmetry. Since chirality depends on configuration, not
conformation, cyclic molecules can be represented as planar structures to facilitate SECTION 2.1
recognition of symmetry elements. These planar structures clearly convey the cis and Configuration
trans relationships between substituents. Scheme 2.1 gives some examples of both
chiral and achiral dimethylcycloalkanes. Note that in several of the compounds there
is both a center and a plane of symmetry. Either element of symmetry ensures that the
molecule is achiral.
Scheme 2.1. Chiral and Achiral Disubstituted Cycloalkanes
Achiral
CH CH CH
CH 3 CH 3 CH 3 3 3
CH 3 CH 3 3 CH 3 CH 3
CH
CH 3 3
Chiral CH 3 CH 3 CH
CH 3 CH 3 CH 3 3
CH CH CH 3
CH 3 CH 3 3 3 CH 3
2.1.7. Configuration at Prochiral Centers
Prochiral centers have two identical ligands, such as two hydrogens, and are
achiral. In many situations, however, these identical ligands are topologically nonequiv-
alent or heterotopic. This occurs when the other two substituents are different. If
either of the identical groups is replaced by a different ligand, a stereogenic center
is created. The two positions are called enantiotopic. The position, which if assigned
a higher priority, gives an R configuration is called pro-R. The position, which if
assigned a higher priority, gives an S configuration is called pro-S. Propane-1,3-diol
is an example of a prochiral molecule. The C(1) and C(3) positions are prochiral, but
the C(2) is not, because its two hydroxymethyl ligands are identical.
HO OH
H H S H R H S
R
Unsymmetrically substituted carbonyl groups are prochiral centers, since addition
of a fourth ligand generates a stereogenic center. These are designated by determining the
Cahn-Ingold-Prelog priority order. The carbonyl group is said to have an re face and an
si face.