Page 155 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 155
135
SECTION 2.1
Configuration
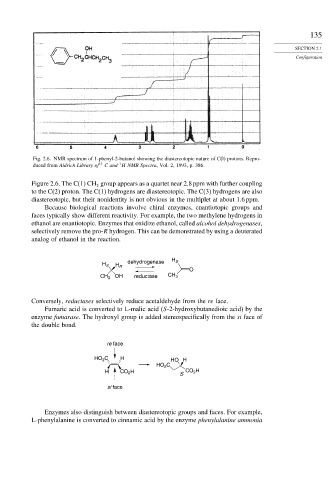
Fig. 2.6. NMR spectrum of 1-phenyl-2-butanol showing the diastereotopic nature of C(l) protons. Repro-
1
duced from Aldrich Library of 13 C and H NMR Spectra, Vol. 2, 1993, p. 386.
Figure 2.6. The C(1) CH group appears as a quartet near 2.8 ppm with further coupling
2
to the C(2) proton. The C(1) hydrogens are diastereotopic. The C(3) hydrogens are also
diastereotopic, but their nonidentity is not obvious in the multiplet at about 1.6 ppm.
Because biological reactions involve chiral enzymes, enantiotopic groups and
faces typically show different reactivity. For example, the two methylene hydrogens in
ethanol are enantiotopic. Enzymes that oxidize ethanol, called alcohol dehydrogenases,
selectively remove the pro-R hydrogen. This can be demonstrated by using a deuterated
analog of ethanol in the reaction.
H
dehydrogenase S
H S H R
O
CH 3 OH reductase CH 3
Conversely, reductases selectively reduce acetaldehyde from the re face.
Fumaric acid is converted to L-malic acid (S-2-hydroxybutanedioic acid) by the
enzyme fumarase. The hydroxyl group is added stereospecifically from the si face of
the double bond.
re face
C H
HO 2 HO H
HO C
2
H CO 2 H CO H
2
S
si face
Enzymes also distinguish between diastereotopic groups and faces. For example,
L-phenylalanine is converted to cinnamic acid by the enzyme phenylalanine ammonia