Page 154 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 154
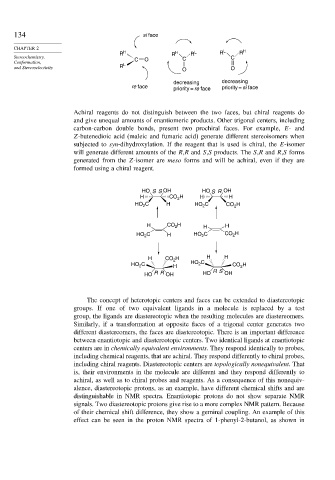
134 si face
CHAPTER 2 L H
R H R H R L R R
Stereochemistry, C O C C
Conformation, L
and Stereoselectivity R O O
decreasing decreasing
re face priority = si face
priority = re face
Achiral reagents do not distinguish between the two faces, but chiral reagents do
and give unequal amounts of enantiomeric products. Other trigonal centers, including
carbon-carbon double bonds, present two prochiral faces. For example, E- and
Z-butenedioic acid (maleic and fumaric acid) generate different stereoisomers when
subjected to syn-dihydroxylation. If the reagent that is used is chiral, the E-isomer
will generate different amounts of the R,R and S,S products. The S,R and R,S forms
generated from the Z-isomer are meso forms and will be achiral, even if they are
formed using a chiral reagent.
HO SS OH HO S R OH
H CO 2 H H H
HO 2 C H HO C CO H
2
2
H CO 2 H H H
HO C H HO C CO 2 H
2
2
H CO H H H
2
HO C H HO 2 C CO 2 H
2
HO R R OH HO R S OH
The concept of heterotopic centers and faces can be extended to diastereotopic
groups. If one of two equivalent ligands in a molecule is replaced by a test
group, the ligands are diastereotopic when the resulting molecules are diastereomers.
Similarly, if a transformation at opposite faces of a trigonal center generates two
different diastereomers, the faces are diastereotopic. There is an important difference
between enantiotopic and diastereotopic centers. Two identical ligands at enantiotopic
centers are in chemically equivalent environments. They respond identically to probes,
including chemical reagents, that are achiral. They respond differently to chiral probes,
including chiral reagents. Diastereotopic centers are topologically nonequivalent. That
is, their environments in the molecule are different and they respond differently to
achiral, as well as to chiral probes and reagents. As a consequence of this nonequiv-
alence, diastereotopic protons, as an example, have different chemical shifts and are
distinguishable in NMR spectra. Enantiotopic protons do not show separate NMR
signals. Two diastereotopic protons give rise to a more complex NMR pattern. Because
of their chemical shift difference, they show a geminal coupling. An example of this
effect can be seen in the proton NMR spectra of 1-phenyl-2-butanol, as shown in