Page 151 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 151
H 131
H
SECTION 2.1
Configuration
H H
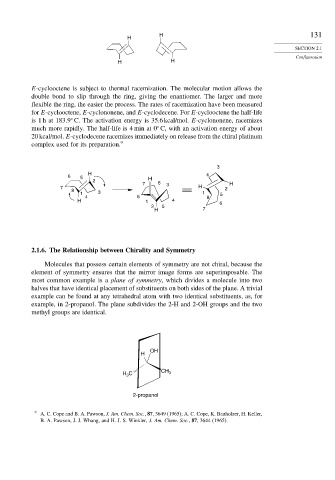
E-cyclooctene is subject to thermal racemization. The molecular motion allows the
double bond to slip through the ring, giving the enantiomer. The larger and more
flexible the ring, the easier the process. The rates of racemization have been measured
for E-cyclooctene, E-cyclononene, and E-cyclodecene. For E-cyclooctene the half-life
is 1 h at 183 9 C. The activation energy is 35.6 kcal/mol. E-cyclononene, racemizes
much more rapidly. The half-life is 4 min at 0 C, with an activation energy of about
20 kcal/mol. E-cyclodecene racemizes immediately on release from the chiral platinum
complex used for its preparation. 9
3
H
6 5 4
2 H
7 6 3 H
7 H 2
8
1 3 1 5
4 8 8
H 1 4 6
2 5
H 7
2.1.6. The Relationship between Chirality and Symmetry
Molecules that possess certain elements of symmetry are not chiral, because the
element of symmetry ensures that the mirror image forms are superimposable. The
most common example is a plane of symmetry, which divides a molecule into two
halves that have identical placement of substituents on both sides of the plane. A trivial
example can be found at any tetrahedral atom with two identical substituents, as, for
example, in 2-propanol. The plane subdivides the 2-H and 2-OH groups and the two
methyl groups are identical.
OH
H
CH
H C 3
3
2-propanol
9
A. C. Cope and B. A. Pawson, J. Am. Chem. Soc., 87, 3649 (1965); A. C. Cope, K. Banholzer, H. Keller,
B. A. Pawson, J. J. Whang, and H. J. S. Winkler, J. Am. Chem. Soc., 87, 3644 (1965).