Page 147 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 147
adjacent substituents pointed in the same direction (in or out) are syn, whereas those 127
pointed in opposite directions are anti.
For molecules with more than one stereogenic center, the enantiomeric pair must SECTION 2.1
have the opposite configuration at each center. The two enantiomeric relationships are Configuration
shown in Figure 2.4. There are four other pairings that do not fulfill this requirement,
but the structures are still stereoisomeric. Molecules that are stereoisomeric but are not
enantiomeric are called diastereomers, and four of these relationships are pointed out in
Figure 2.4. Molecules that are diastereomeric have the same constitution (connectivity)
but differ in configuration at one or more of the stereogenic centers. The positions in
two diastereomers that have different configurations are called epimeric. For example,
the anti-2R,3R and syn-2R,3S stereoisomers have the same configuration at C(2), but
are epimeric at C(3). There is nothing unique about the way in which the molecules
in Figure 2.4 are positioned, except for the conventional depiction of the extended
chain horizontally. For example, the three other representations below also depict the
anti-2R,3S stereoisomer.
OH OH H OH OH H
O
HO O OH O OH HO O
OH H H OH OH OH
anti 2R,3S anti 2R,3S anti 2R,3S anti 2R,3S
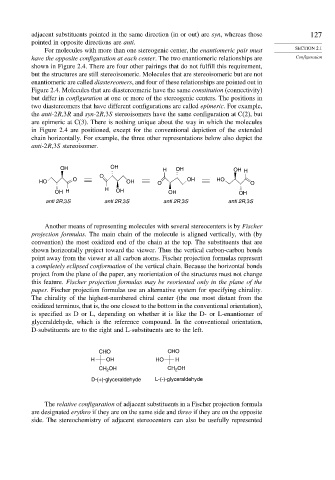
Another means of representing molecules with several stereocenters is by Fischer
projection formulas. The main chain of the molecule is aligned vertically, with (by
convention) the most oxidized end of the chain at the top. The substituents that are
shown horizontally project toward the viewer. Thus the vertical carbon-carbon bonds
point away from the viewer at all carbon atoms. Fischer projection formulas represent
a completely eclipsed conformation of the vertical chain. Because the horizontal bonds
project from the plane of the paper, any reorientation of the structures must not change
this feature. Fischer projection formulas may be reoriented only in the plane of the
paper. Fischer projection formulas use an alternative system for specifying chirality.
The chirality of the highest-numbered chiral center (the one most distant from the
oxidized terminus, that is, the one closest to the bottom in the conventional orientation),
is specified as D or L, depending on whether it is like the D- or L-enantiomer of
glyceraldehyde, which is the reference compound. In the conventional orientation,
D-substituents are to the right and L-substituents are to the left.
CHO CHO
H OH HO H
CH OH CH 2 OH
2
D-(+)-glyceraldehyde L-(-)-glyceraldehyde
The relative configuration of adjacent substituents in a Fischer projection formula
are designated erythro if they are on the same side and threo if they are on the opposite
side. The stereochemistry of adjacent stereocenters can also be usefully represented