Page 142 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 142
122 2.1.3. Configuration at Tetrahedral Atoms
CHAPTER 2 Carbon and other atoms with sp 3 hybridization have approximately tetra-
Stereochemistry, hedral geometry. With the exception of small deviations in bond angles, each of
Conformation, the substituents is in a geometrically equivalent position. Nevertheless, there is
and Stereoselectivity
an important stereochemical feature associated with tetrahedral centers. If all four
substituents are different, they can be arranged in two different ways. The two different
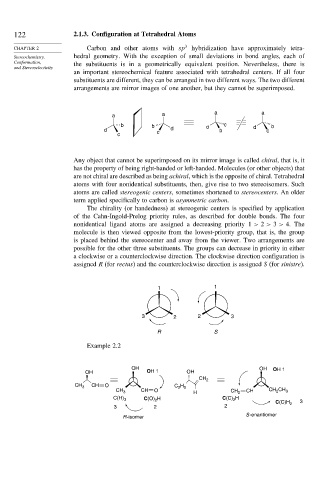
arrangements are mirror images of one another, but they cannot be superimposed.
a a a a
b b d c d b
d c d b c
c
Any object that cannot be superimposed on its mirror image is called chiral, that is, it
has the property of being right-handed or left-handed. Molecules (or other objects) that
are not chiral are described as being achiral, which is the opposite of chiral. Tetrahedral
atoms with four nonidentical substituents, then, give rise to two stereoisomers. Such
atoms are called stereogenic centers, sometimes shortened to stereocenters. An older
term applied specifically to carbon is asymmetric carbon.
The chirality (or handedness) at stereogenic centers is specified by application
of the Cahn-Ingold-Prelog priority rules, as described for double bonds. The four
nonidentical ligand atoms are assigned a decreasing priority 1 > 2 > 3 > 4. The
molecule is then viewed opposite from the lowest-priority group, that is, the group
is placed behind the stereocenter and away from the viewer. Two arrangements are
possible for the other three substituents. The groups can decrease in priority in either
a clockwise or a counterclockwise direction. The clockwise direction configuration is
assigned R (for rectus) and the counterclockwise direction is assigned S (for sinistre).
1 1
3 2 2 3
R S
Example 2.2
OH OH OH 1
OH OH 1 OH
CH 2
CH
CH 3 O C 2 H 5
CH O
H CH 2
CH 3 CH CH 2 CH 3
C(O) 2 H C(C) 2 H
C(H) 3 3
C(C)H 2
3 2 2
R -isomer S -enantiomer