Page 141 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 141
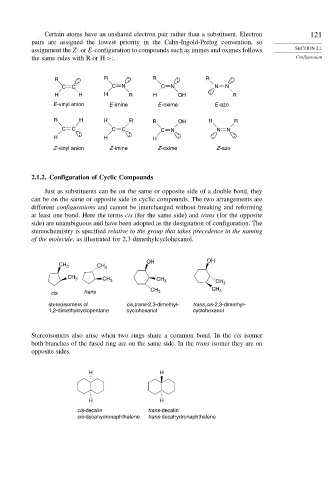
Certain atoms have an unshared electron pair rather than a substituent. Electron 121
pairs are assigned the lowest priority in the Cahn-Ingold-Prelog convention, so
assignment the Z-or E-configuration to compounds such as imines and oximes follows SECTION 2.1
the same rules with R or H >:. Configuration
R : R : R : R :
C C C N C N N N
H H H R H OH : R
E -vinyl anion E -imine E -oxime E -azo
R H R R R OH R R
C C C C C N N N
: : : :
H H H :
Z -vinyl anion Z -imine Z -oxime Z -azo
2.1.2. Configuration of Cyclic Compounds
Just as substituents can be on the same or opposite side of a double bond, they
can be on the same or opposite side in cyclic compounds. The two arrangements are
different configurations and cannot be interchanged without breaking and reforming
at least one bond. Here the terms cis (for the same side) and trans (for the opposite
side) are unambiguous and have been adopted as the designation of configuration. The
stereochemistry is specified relative to the group that takes precedence in the naming
of the molecule, as illustrated for 2,3-dimethylcyclohexanol.
OH OH
CH 3 CH 3
CH 3 CH 3 CH 3 CH 3
CH CH 3
cis trans 3
stereoisomers of cis,trans-2,3-dimethyl- trans,cis-2,3-dimethyl-
1,2-dimethylcyclopentane cyclohexanol cyclohexanol
Stereoisomers also arise when two rings share a common bond. In the cis isomer
both branches of the fused ring are on the same side. In the trans isomer they are on
opposite sides.
H H
H H
cis-decalin trans-decalin
cis-decahydronaphthalene trans-decahydronaphthalene