Page 149 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 149
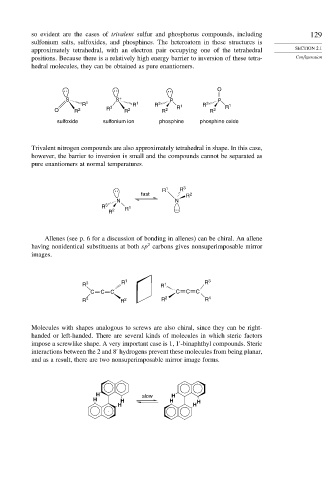
so evident are the cases of trivalent sulfur and phosphorus compounds, including 129
sulfonium salts, sulfoxides, and phosphines. The heteroatom in these structures is
approximately tetrahedral, with an electron pair occupying one of the tetrahedral SECTION 2.1
positions. Because there is a relatively high energy barrier to inversion of these tetra- Configuration
hedral molecules, they can be obtained as pure enantiomers.
O
: : :
S S + P P
R 1 R 1 R 3 1 R 3 R 1
O R 2 R 3 R 2 R 2 R R 2
sulfoxide sulfonium ion phosphine phosphine oxide
Trivalent nitrogen compounds are also approximately tetrahedral in shape. In this case,
however, the barrier to inversion is small and the compounds cannot be separated as
pure enantiomers at normal temperatures.
: R 1 R 3
fast R 2
N N
R 3 R 1
R 2
Allenes (see p. 6 for a discussion of bonding in allenes) can be chiral. An allene
2
having nonidentical substituents at both sp carbons gives nonsuperimposable mirror
images.
R 3 R 1 R 1 R 3
C C C C C C
R 4 R 2 R 2 R 4
Molecules with shapes analogous to screws are also chiral, since they can be right-
handed or left-handed. There are several kinds of molecules in which steric factors
impose a screwlike shape. A very important case is 1 1 -binaphthyl compounds. Steric
interactions between the 2 and 8 hydrogens prevent these molecules from being planar,
and as a result, there are two nonsuperimposable mirror image forms.
H slow H
H H H H
H H