Page 161 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 161
141
SECTION 2.1
Configuration
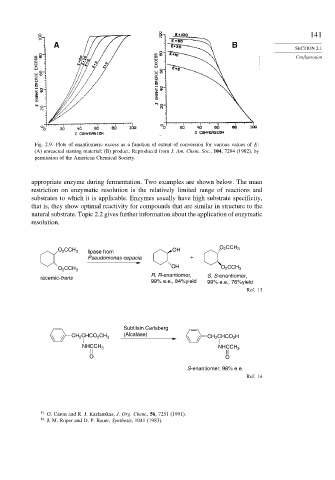
Fig. 2.9. Plots of enantiomeric excess as a function of extent of conversion for various values of E:
(A) unreacted starting material; (B) product. Reproduced from J. Am. Chem. Soc., 104, 7294 (1982), by
permission of the American Chemical Society.
appropriate enzyme during fermentation. Two examples are shown below. The main
restriction on enzymatic resolution is the relatively limited range of reactions and
substrates to which it is applicable. Enzymes usually have high substrate specificity,
that is, they show optimal reactivity for compounds that are similar in structure to the
natural substrate. Topic 2.2 gives further information about the application of enzymatic
resolution.
O CCH 3 lipase from OH O 2 CCH 3
2
Pseudomonas cepacia +
O CCH 3 OH O 2 CCH 3
2
R, R-enantiomer, S, S-enantiomer,
racemic-trans
99% e.e., 84%yield 99% e.e., 76%yield
Ref. 13
Subtilsin Carlsberg
CH CHCO CH 3 (Alcalase) CH 2 CHCO 2 H
2
2
NHCCH 3 NHCCH 3
O O
S-enantiomer, 98% e.e.
Ref. 14
13 G. Caron and R. J. Kazlauskas, J. Org. Chem., 56, 7251 (1991).
14
J. M. Roper and D. P. Bauer, Synthesis, 1041 (1983).