Page 166 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 166
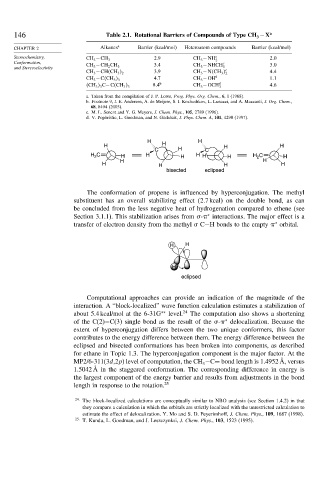
146 Table 2.1. Rotational Barriers of Compounds of Type CH −X a
3
CHAPTER 2 Alkanes a Barrier (kcal/mol) Heteroatom compounds Barrier (kcal/mol)
Stereochemistry, 2.9 CH 3 −NH c 2 0
CH 3 −CH 3 2
Conformation, 3.4 CH 3 −NHCH c 3 0
and Stereoselectivity CH 3 −CH 2 CH 3 3 c
CH 3 −CH CH 3 2 3.9 CH 3 −N CH 3 2 4 4
4.7 CH 3 −OH d 1 1
CH 3 −C CH 3 3
8 4 b CH 3 −OCH d 4 6
CH 3 3 C−C CH 3 3 3
a. Taken from the compilation of J. P. Lowe, Prog. Phys. Org. Chem., 6, 1 (1968).
b. Footnote 9, J. E. Andersen, A. de Meijere, S. I. Kozhushkov, L. Lunazzi, and A. Mazzanti, J. Org. Chem.,
68, 8494 (2003).
c. M. L. Senent and Y. G. Meyers, J. Chem. Phys., 105, 2789 (1996).
d. V. Pophristic, L. Goodman, and N. Guchhait, J. Phys. Chem. A, 101, 4290 (1997).
H H
H H H H
C H C
H 2 C H H H H H H H 2 C H
H H
H H H H
bisected eclipsed
The conformation of propene is influenced by hyperconjugation. The methyl
substituent has an overall stabilizing effect (2.7 kcal) on the double bond, as can
be concluded from the less negative heat of hydrogenation compared to ethene (see
∗
Section 3.1.1). This stabilization arises from - interactions. The major effect is a
∗
transfer of electron density from the methyl C−H bonds to the empty orbital.
H H
H
eclipsed
Computational approaches can provide an indication of the magnitude of the
interaction. A “block-localized” wave function calculation estimates a stabilization of
about 5.4 kcal/mol at the 6-31G ∗∗ level. 24 The computation also shows a shortening
of the C(2)−C(3) single bond as the result of the - delocalization. Because the
∗
extent of hyperconjugation differs between the two unique conformers, this factor
contributes to the energy difference between them. The energy difference between the
eclipsed and bisected conformations has been broken into components, as described
for ethane in Topic 1.3. The hyperconjugation component is the major factor. At the
MP2/6-311(3d,2p) level of computation, the CH −C= bond length is 1.4952 Å, versus
3
1.5042 Å in the staggered conformation. The corresponding difference in energy is
the largest component of the energy barrier and results from adjustments in the bond
length in response to the rotation. 25
24 The block-localized calculations are conceptually similar to NBO analysis (see Section 1.4.2) in that
they compare a calculation in which the orbitals are strictly localized with the unrestricted calculation to
estimate the effect of delocalization. Y. Mo and S. D. Peyerimhoff, J. Chem. Phys., 109, 1687 (1998).
25
T. Kundu, L. Goodman, and J. Leszczynksi, J. Chem. Phys., 103, 1523 (1995).