Page 221 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 221
reactant is a distinct step in the mechanism and that there are attractive interactions 201
between the catalyst ligands and the reactant. 171 The ligands do not function only by
steric exclusion, but contribute to the net stabilization of the TS. (3) The aromatic SECTION 2.5
linker groups determine the size of the binding pocket; the ethyl groups on the quinu- Enantioselective
Reactions
clidine ring have differing orientations in the DHQD and DHQ catalysts and are a more
integral part of the pocket in the DHQD system. (4) The concerted 2+3 cycloaddition
mechanism for the actual oxidation step is energetically preferable to an alternative
two-step mechanism involving 2+2 cycloaddition. 172
O O
O O
O O O Os
O O O Os O O O Os
Os Os O O
O O O
O O
2 + 3 2 + 2
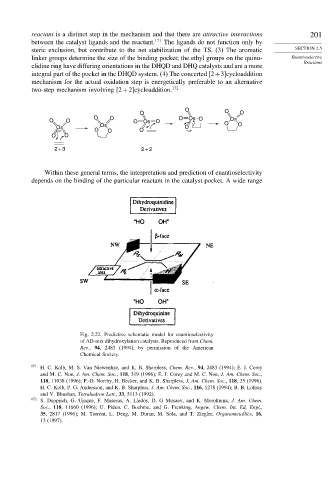
Within these general terms, the interpretation and prediction of enantioselectivity
depends on the binding of the particular reactant in the catalyst pocket. A wide range
Fig. 2.22. Predictive schematic model for enantioselectivity
of AD-mix dihydroxylation catalysts. Reproduced from Chem.
Rev., 94, 2483 (1994), by permission of the American
Chemical Society.
171 H. C. Kolb, M. S. Van Niewenhze, and K. B. Sharpless, Chem. Rev., 94, 2483 (1994); E. J. Corey
and M. C. Noe, J. Am. Chem. Soc., 118, 319 (1996); E. J. Corey and M. C. Noe, J. Am. Chem. Soc.,
118, 11038 (1996); P.-O. Norrby, H. Becker, and K. B. Sharpless, J. Am. Chem. Soc., 118, 35 (1996).
H. C. Kolb, P. G. Andersson, and K. B. Sharpless, J. Am. Chem. Soc., 116, 1278 (1994); B. B. Lohray
and V. Bhushan, Tetrahedron Lett., 33, 5113 (1992).
172
S. Dapprich, G. Ujaque, F. Maseras, A. Lledos, D. G Musaev, and K. Morokuma, J. Am. Chem.
Soc., 118, 11660 (1996); U. Pidun, C. Boehme, and G. Frenking, Angew. Chem. Int. Ed. Engl.,
35, 2817 (1996); M. Torrent, L. Deng, M. Duran, M. Sola, and T. Ziegler, Organometallics, 16,
13 (1997).