Page 223 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 223
203
SECTION 2.5
Enantioselective
Reactions
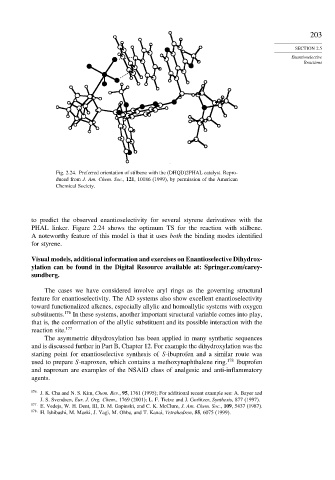
Fig. 2.24. Preferred orientation of stilbene with the (DHQD)2PHAL catalyst. Repro-
duced from J. Am. Chem. Soc., 121, 10186 (1999), by permission of the American
Chemical Society.
to predict the observed enantioselectivity for several styrene derivatives with the
PHAL linker. Figure 2.24 shows the optimum TS for the reaction with stilbene.
A noteworthy feature of this model is that it uses both the binding modes identified
for styrene.
Visual models, additional information and exercises on Enantioselective Dihydrox-
ylation can be found in the Digital Resource available at: Springer.com/carey-
sundberg.
The cases we have considered involve aryl rings as the governing structural
feature for enantioselectivity. The AD systems also show excellent enantioselectivity
toward functionalized alkenes, especially allylic and homoallylic systems with oxygen
substituents. 176 In these systems, another important structural variable comes into play,
that is, the conformation of the allylic substituent and its possible interaction with the
reaction site. 177
The asymmetric dihydroxylation has been applied in many synthetic sequences
and is discussed further in Part B, Chapter 12. For example the dihydroxylation was the
starting point for enantioselective synthesis of S-ibuprofen and a similar route was
used to prepare S-naproxen, which contains a methoxynaphthalene ring. 178 Ibuprofen
and naproxen are examples of the NSAID class of analgesic and anti-inflammatory
agents.
176
J. K. Cha and N. S. Kim, Chem. Rev., 95, 1761 (1995); For additional recent example see: A. Bayer and
J. S. Svendsen, Eur. J. Org. Chem., 1769 (2001); L. F. Tietze and J. Gorlitzer, Synthesis, 877 (1997).
177 E. Vedejs, W. H. Dent, III, D. M. Gapinski, and C. K. McClure, J. Am. Chem. Soc., 109, 5437 (1987).
178
H. Ishibashi, M. Maeki, J. Yagi, M. Ohba, and T. Kanai, Tetrahedron, 55, 6075 (1999).