Page 225 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 225
can be expressed in terms of the free-energy differences resulting from the individual 205
preferences G ∗ and G ∗ plus any incremental change resulting from the combi-
1 2
nation G ∗ . SECTION 2.6
12
Double
Stereodifferentiation:
∗
∗
G ∗ matched = G + G + G ∗ 12 Reinforcing and
1
2
Competing
∗
∗
G ∗ mismatched = G − G + G ∗ 12 Stereoselectivity
2
1
An example of such a situation is an aldol addition reaction in which one carbonyl
compound acts as the nucleophile and the other as the electrophile.
CH 3 CH
CH 3 3
CH 2 H
R R R R
–
O M + O O OH new stereocenter
CH 3
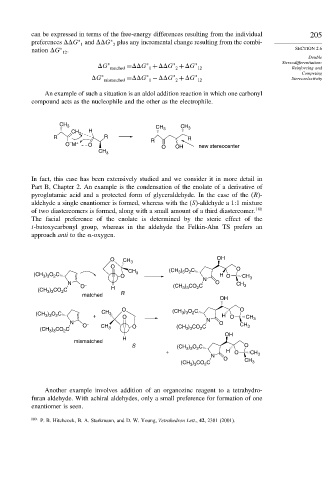
In fact, this case has been extensively studied and we consider it in more detail in
Part B, Chapter 2. An example is the condensation of the enolate of a derivative of
pyroglutamic acid and a protected form of glyceraldehyde. In the case of the (R)-
aldehyde a single enantiomer is formed, whereas with the (S)-aldehyde a 1:1 mixture
of two diastereomers is formed, along with a small amount of a third diastereomer. 180
The facial preference of the enolate is determined by the steric effect of the
t-butoxycarbonyl group, whereas in the aldehyde the Felkin-Ahn TS prefers an
approach anti to the -oxygen.
O CH 3 OH
O O
CH (CH ) O C
2
3 3
(CH ) O C O 3 H O CH
2
3 3
N 3
N O – ) CO C O CH 3
(CH ) CO C H (CH 3 3 2
3 3
2
matched R
OH
O (CH ) O C O
(CH ) O C + CH 3 O 3 3 2 H O CH
3 3
2
N N O 3
O – CH O (CH ) CO C CH 3
(CH ) CO C 3 3 3 2
3 3
2
OH
H
mismatched
S (CH ) O C O
2
3 3
+ H O CH
N 3
O CH
) CO C 3
(CH 3 3 2
Another example involves addition of an organozinc reagent to a tetrahydro-
furan aldehyde. With achiral aldehydes, only a small preference for formation of one
enantiomer is seen.
180
P. B. Hitchcock, B. A. Starkmann, and D. W. Young, Tetrahedron Lett., 42, 2381 (2001).