Page 228 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 228
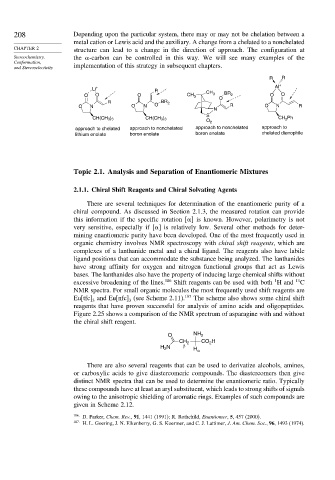
208 Depending upon the particular system, there may or may not be chelation between a
metal cation or Lewis acid and the auxiliary. A change from a chelated to a nonchelated
CHAPTER 2 structure can lead to a change in the direction of approach. The configuration at
Stereochemistry, the -carbon can be controlled in this way. We will see many examples of the
Conformation,
and Stereoselectivity implementation of this strategy in subsequent chapters.
R R
Li + R Al +
O O O CH CH 3 BR 2 O O
3
O
R BR 2
O N O N O R O N R
N
S
CH(CH 3) 3 CH(CH 3) 3 CH Ph
2
O 2
approach to chelated approach to nonchelated approach to nonchelated approach to
lithium enolate boron enolate boron enolate chelated dienophile
Topic 2.1. Analysis and Separation of Enantiomeric Mixtures
2.1.1. Chiral Shift Reagents and Chiral Solvating Agents
There are several techniques for determination of the enantiomeric purity of a
chiral compound. As discussed in Section 2.1.3, the measured rotation can provide
this information if the specific rotation is known. However, polarimetry is not
very sensitive, especially if is relatively low. Several other methods for deter-
mining enantiomeric purity have been developed. One of the most frequently used in
organic chemistry involves NMR spectroscopy with chiral shift reagents, which are
complexes of a lanthanide metal and a chiral ligand. The reagents also have labile
ligand positions that can accommodate the substance being analyzed. The lanthanides
have strong affinity for oxygen and nitrogen functional groups that act as Lewis
bases. The lanthanides also have the property of inducing large chemical shifts without
1
excessive broadening of the lines. 186 Shift reagents can be used with both H and 13 C
NMR spectra. For small organic molecules the most frequently used shift reagents are
Eu[tfc] and Eu[nfc] (see Scheme 2.11). 187 The scheme also shows some chiral shift
3 3
reagents that have proven successful for analysis of amino acids and oligopeptides.
Figure 2.25 shows a comparison of the NMR spectrum of asparagine with and without
the chiral shift reagent.
O NH 2
CH 2 CO H
2
N β
H 2 H α
There are also several reagents that can be used to derivatize alcohols, amines,
or carboxylic acids to give diastereomeric compounds. The diastereomers then give
distinct NMR spectra that can be used to determine the enantiomeric ratio. Typically
these compounds have at least an aryl substituent, which leads to strong shifts of signals
owing to the anisotropic shielding of aromatic rings. Examples of such compounds are
given in Scheme 2.12.
186 D. Parker, Chem. Rev., 91, 1441 (1991); R. Rothchild, Enantiomer, 5, 457 (2000).
187
H. L. Goering, J. N. Eikenberry, G. S. Koermer, and C. J. Lattimer, J. Am. Chem. Soc., 96, 1493 (1974).