Page 231 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 231
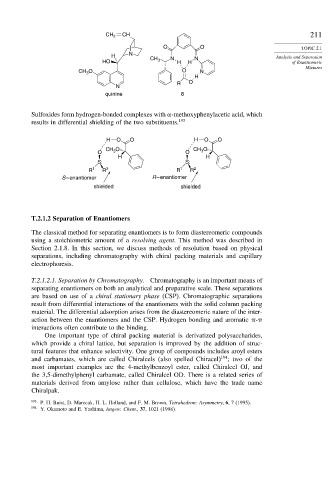
CH
CH 2 211
O O TOPIC 2.1
N
H CH N N Analysis and Separation
HO 3 H H of Enantiomeric
Mixtures
CH O O N
3
H
R O
N
quinine 8
Sulfoxides form hydrogen-bonded complexes with -methoxyphenylacetic acid, which
results in differential shielding of the two substituents. 193
H O O H O O
3
O CH 3 O O CH O
H H
S: S:
R 1 R 2 R 1 R 2
S – enantiomer R – enantiomer
shielded shielded
T.2.1.2 Separation of Enantiomers
The classical method for separating enantiomers is to form diastereomeric compounds
using a stoichiometric amount of a resolving agent. This method was described in
Section 2.1.8. In this section, we discuss methods of resolution based on physical
separations, including chromatography with chiral packing materials and capillary
electrophoresis.
T.2.1.2.1. Separation by Chromatography. Chromatography is an important means of
separating enantiomers on both an analytical and preparative scale. These separations
are based on use of a chiral stationary phase (CSP). Chromatographic separations
result from differential interactions of the enantiomers with the solid column packing
material. The differential adsorption arises from the diastereomeric nature of the inter-
action between the enantiomers and the CSP. Hydrogen bonding and aromatic -
interactions often contribute to the binding.
One important type of chiral packing material is derivatized polysaccharides,
which provide a chiral lattice, but separation is improved by the addition of struc-
tural features that enhance selectivity. One group of compounds includes aroyl esters
and carbamates, which are called Chiralcels (also spelled Chiracel) 194 ; two of the
most important examples are the 4-methylbenzoyl ester, called Chiralcel OJ, and
the 3,5-dimethylphenyl carbamate, called Chiralcel OD. There is a related series of
materials derived from amylose rather than cellulose, which have the trade name
Chiralpak.
193 P. H. Buist, D. Marecak, H. L. Holland, and F. M. Brown, Tetrahedron: Asymmetry, 6, 7 (1995).
194
Y. Okamoto and E. Yashima, Angew. Chem., 37, 1021 (1998).