Page 236 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 236
216 resolved must be an acceptable substrate for the enzyme. If not, there will be no reaction
with either enantiomer. The types of reactions that are suitable for enzymatic resolu-
CHAPTER 2 tions are somewhat limited. The most versatile enzymes—esterases and lipases—
Stereochemistry, catalyze formation or hydrolysis of esters. There are also enzymes that catalyze amide
Conformation,
and Stereoselectivity formation and hydrolysis, which can be broadly categorized as acylases or amidases.
We also discuss epoxide hydrolases, which open epoxide rings. Another important
family is the oxido-reductases, which interconvert alcohols and carbonyl compound
by oxidation and reduction.
T.2.2.1. Lipases and Esterases
The most widely applied enzymes for resolution are lipases and esterases, which
can catalyze either the hydrolysis or the formation of esters. 209 The natural function
of these enzymes is to catalyze hydrolysis of fatty acid esters of glycerol. There are a
number of such enzymes that are commercially available. A very important property of
these esterases and lipases is that they can accept a fairly wide variety of molecules as
substrates. They are also adaptable for use in organic solvents, which further enhances
their practical utility. 210
The esterases and lipases are members of a still larger group of enzymes that
catalyze acyl transfer, either in the direction of solvolysis or by acylation of the
substrate. Both types of enzymes are called hydrolases. In water, hydrolysis occurs,
but in the presence of alcohols, transesterification can occur. Reactions in the acylation
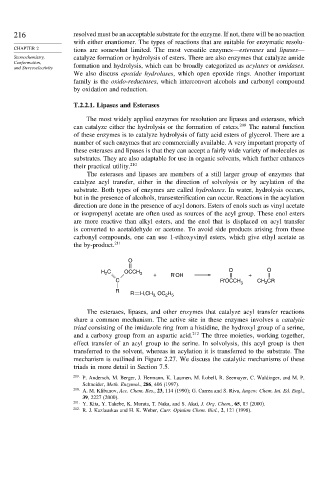
direction are done in the presence of acyl donors. Esters of enols such as vinyl acetate
or isopropenyl acetate are often used as sources of the acyl group. These enol esters
are more reactive than alkyl esters, and the enol that is displaced on acyl transfer
is converted to acetaldehyde or acetone. To avoid side products arising from these
carbonyl compounds, one can use 1-ethoxyvinyl esters, which give ethyl acetate as
the by-product. 211
O
H C OCCH 3 + R'OH O + O
2
C R'OCCH CR
3 CH 3
R
R H,CH OC H
2 5
3,
The esterases, lipases, and other enzymes that catalyze acyl transfer reactions
share a common mechanism. The active site in these enzymes involves a catalytic
triad consisting of the imidazole ring from a histidine, the hydroxyl group of a serine,
and a carboxy group from an aspartic acid. 212 The three moieties, working together,
effect transfer of an acyl group to the serine. In solvolysis, this acyl group is then
transferred to the solvent, whereas in acylation it is transferred to the substrate. The
mechanism is outlined in Figure 2.27. We discuss the catalytic mechanisms of these
triads in more detail in Section 7.5.
209
P. Andersch, M. Berger, J. Hermann, K. Laumen, M. Lobell, R. Seemayer, C. Waldinger, and M. P.
Schneider, Meth. Enzymol., 286, 406 (1997).
210 A. M. Klibanov, Acc. Chem. Res., 23, 114 (1990); G. Carrea and S. Riva, Angew. Chem. Int. Ed. Engl.,
39, 2227 (2000).
211
Y. Kita, Y. Takebe, K. Murata, T. Naka, and S. Akai, J. Org. Chem., 65, 83 (2000).
212 R. J. Kazlauskas and H. K. Weber, Curr. Opinion Chem. Biol., 2, 121 (1998).