Page 237 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 237
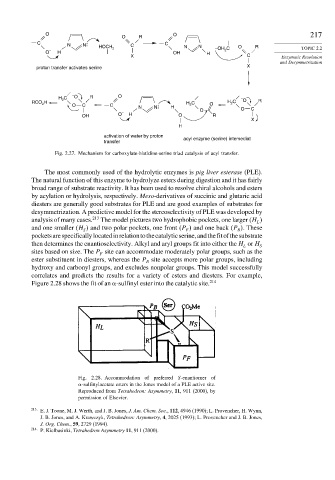
O O 217
O R
C C
N N HOCH C N N –OH C O R TOPIC 2.2
O – H 2 HO H 2
X C Enzymatic Resolution
and Desymmetrization
X
proton transfer activates serine
– O O
C R
H 2 – O R
RCO H H 2 C O H C
2
2
C
C
O
N N H
O O C
OH O – H O R
X
H
activation of water by proton
acyl enzyme (serine) intemediat
transfer
Fig. 2.27. Mechanism for carboxylate-histidine-serine triad catalysis of acyl transfer.
The most commonly used of the hydrolytic enzymes is pig liver esterase (PLE).
The natural function of this enzyme to hydrolyze esters during digestion and it has fairly
broad range of substrate reactivity. It has been used to resolve chiral alcohols and esters
by acylation or hydrolysis, respectively. Meso-derivatives of succinic and glutaric acid
diesters are generally good substrates for PLE and are good examples of substrates for
desymmetrization. A predictive model for the stereoselectivity of PLE was developed by
analysis of many cases. 213 The model pictures two hydrophobic pockets, one larger H
L
and one smaller H and two polar pockets, one front P and one back P . These
S
F
B
pocketsarespecificallylocatedinrelationtothecatalyticserine,andthefitofthesubstrate
then determines the enantioselectivity. Alkyl and aryl groups fit into either the H or H S
L
sites based on size. The P site can accommodate moderately polar groups, such as the
F
ester substituent in diesters, whereas the P site accepts more polar groups, including
B
hydroxy and carbonyl groups, and excludes nonpolar groups. This model successfully
correlates and predicts the results for a variety of esters and diesters. For example,
Figure 2.28 shows the fit of an -sulfinyl ester into the catalytic site. 214
Fig. 2.28. Accommodation of preferred S-enantiomer of
-sulfinylacetate esters in the Jones model of a PLE active site.
Reproduced from Tetrahedron: Asymmetry, 11, 911 (2000), by
permission of Elsevier.
213 E. J. Toone, M. J. Werth, and J. B. Jones, J. Am. Chem. Soc., 112, 4946 (1990); L. Provencher, H. Wynn,
J. B. Jones, and A. Krawczyk, Tetrahedron: Asymmetry, 4, 2025 (1993); L. Provencher and J. B. Jones,
J. Org. Chem., 59, 2729 (1994).
214
P. Kielbasinki, Tetrahedron Asymmetry 11, 911 (2000).