Page 239 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 239
stereoselectivity of the reaction is determined by the relative fit in the binding site of 219
the esterases.
Lipases are another important group of hydrolases. The most commonly used TOPIC 2.2
example is porcine pancreatic lipase (PPL). Lipases tend to function best at or above Enzymatic Resolution
and Desymmetrization
the solubility limit of the hydrophobic substrate. In the presence of water, the substrate
forms an insoluble phase (micelles); the concentration at which this occurs is called the
critical micellar concentration. The enzyme is activated by a conformational change
that occurs in the presence of the micelles and results in the opening of the active site.
Lipases work best in solvents that can accommodate this activation process. 215 PPL
is often used as a relatively crude preparation called “pancreatin” or “steapsin.” The
active site in PPL has not been as precisely described as the one for PLE. There are
currently two different models, but they sometimes make contradictory predictions. 216
It has been suggested that the dominant factors in binding are the hydrophobic and
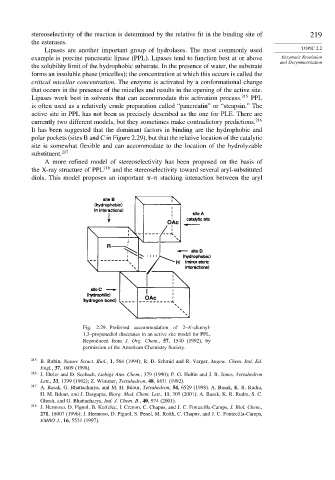
polar pockets (sites B and C in Figure 2.29), but that the relative location of the catalytic
site is somewhat flexible and can accommodate to the location of the hydrolyzable
substituent. 217
A more refined model of stereoselectivity has been proposed on the basis of
the X-ray structure of PPL 218 and the stereoselectivity toward several aryl-substituted
diols. This model proposes an important - stacking interaction between the aryl
Fig. 2.29. Preferred accommodation of 2–E-alkenyl-
1,3–propanediol diacetates in an active site model for PPL.
Reproduced from J. Org. Chem., 57, 1540 (1992), by
permission of the American Chemistry Society.
215 B. Rubin, Nature Struct. Biol., 1, 568 (1994); R. D. Schmid and R. Verger, Angew. Chem. Intl. Ed.
Engl., 37, 1609 (1998).
216
J. Ehrler and D. Seebach, Liebigs Ann. Chem., 379 (1990); P. G. Hultin and J. B. Jones, Tetrahedron
Lett., 33, 1399 (1992); Z. Wimmer, Tetrahedron, 48, 8431 (1992).
217 A. Basak, G. Bhattacharya, and M. H. Bdour, Tetrahedron, 54, 6529 (1998). A. Basak, K. R. Rudra,
H. M. Bdour, and J. Dasgupta, Biorg. Med. Chem. Lett., 11, 305 (2001); A. Basak, K. R. Rudra, S. C.
Ghosh, and G. Bhattacharya, Ind. J. Chem. B., 40, 974 (2001).
218
J. Hermoso, D. Pignol, B. Kerfelec, I. Crenon, C. Chapus, and J. C. Fontecilla-Camps, J. Biol. Chem.,
271, 18007 (1996); J. Hermoso, D. Pignol, S. Penel, M. Roith, C. Chapus, and J. C. Fontecilla-Camps,
EMBO J., 16, 5531 (1997).