Page 243 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 243
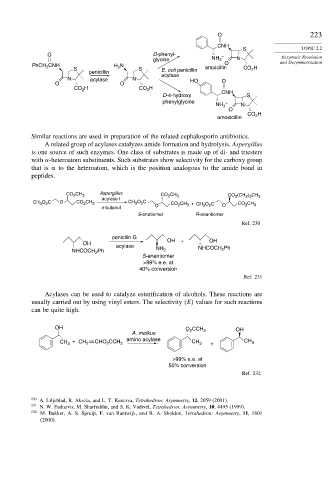
O 223
CNH
S TOPIC 2.2
O D-phenyl- + Enzymatic Resolution
glycine NH 3 N and Desymmetrization
PhCH 2 CNH H 2 N O
S S E. coli penicillin ampicillin CO 2 H
penicillin
acylase
N acylase N HO O
O O
CO 2 H CO 2 H
CNH
D-4-hydroxy S
phenylglycine +
NH 3 N
O
CO 2 H
amoxicillin
Similar reactions are used in preparation of the related cephalosporin antibiotics.
A related group of acylases catalyzes amide formation and hydrolysis. Aspergillus
is one source of such enzymes. One class of substrates is made up of di- and triesters
with -heteroatom substituents. Such substrates show selectivity for the carboxy group
that is to the heteroatom, which is the position analogous to the amide bond in
peptides.
Aspergillus
CO 2 CH 3 CO CH 3 CO 2 (CH ) CH 3
2
2 3
acylase I
CH O C O CO CH 3 CH O C CO CH + CH O C O CO CH 3
3 2
3 2
2
3
2
2
n-butanol O 3 2
S-enatiomer R-enantiomer
Ref. 230
penicilin G OH
OH + OH
acylase NH NHCOCH Ph
NHCOCH 2 Ph 2 2
S-enantiomer
>99% e.e. at
40% conversion
Ref. 231
Acylases can be used to catalyze esterification of alcohols. These reactions are
usually carried out by using vinyl esters. The selectivity E values for such reactions
can be quite high.
OH O CCH OH
A. melkus 2 3
CH + CH 2 CHO CCH 3 amino acylase CH 3 + CH 3
3
2
>99% e.e. at
50% conversion
Ref. 232
230
A. Liljeblad, R. Aksela, and L. T. Kanerva, Tetrahedron: Asymmetry, 12, 2059 (2001).
231 N. W. Fadnavis, M. Sharfuddin, and S. K. Vadivel, Tetrahedron: Asymmetry, 10, 4495 (1999).
232
M. Bakker, A. S. Spruijt, F. van Rantwijk, and R. A. Sheldon, Tetrahedron: Asymmetry, 11, 1801
(2000).