Page 245 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 245
225
TOPIC 2.2
Enzymatic Resolution
and Desymmetrization
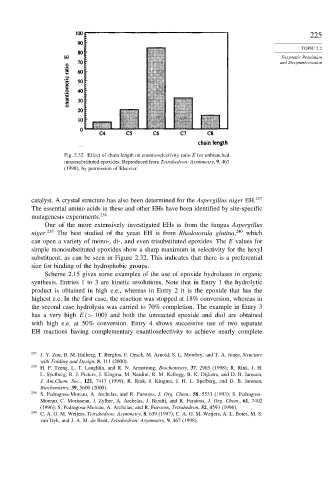
Fig. 2.32. Effect of chain length on enantioselectivity ratio E for unbranched
monosubstituted epoxides. Reproduced from Tetrahedron: Asymmetry, 9, 467
(1998), by permission of Elsevier.
catalyst. A crystal structure has also been determined for the Aspergillus niger EH. 237
The essential amino acids in these and other EHs have been identified by site-specific
mutagenesis experiments. 238
One of the more extensively investigated EHs is from the fungus Aspergillus
niger. 239 The best studied of the yeast EH is from Rhodotorula glutina, 240 which
can open a variety of mono-, di-, and even trisubstituted epoxides. The E values for
simple monosubstituted epoxides show a sharp maximum in selectivity for the hexyl
substituent, as can be seen in Figure 2.32. This indicates that there is a preferential
size for binding of the hydrophobic groups.
Scheme 2.15 gives some examples of the use of epoxide hydrolases in organic
synthesis. Entries 1 to 3 are kinetic resolutions. Note that in Entry 1 the hydrolytic
product is obtained in high e.e., whereas in Entry 2 it is the epoxide that has the
highest e.e. In the first case, the reaction was stopped at 18% conversion, whereas in
the second case hydrolysis was carried to 70% completion. The example in Entry 3
has a very high E > 100 and both the unreacted epoxide and diol are obtained
with high e.e. at 50% conversion. Entry 4 shows successive use of two separate
EH reactions having complementary enantioselectivity to achieve nearly complete
237 J. Y. Zou, B. M. Halberg, T. Bergfos, F. Oesch, M. Arnold, S. L. Mowbry, and T. A. Jones, Structure
with Folding and Design, 8, 111 (2000).
238
H. F. Tzeng, L. T. Laughlin, and R. N. Armstrong, Biochemistry, 37, 2905 (1998); R. Rink, J. H.
L. Spelberg, R. J. Pieters, J. Kingma, M. Nardini, R. M. Kellogg, B. K. Dijkstra, and D. B. Janssen,
J. Am.Chem. Soc., 121, 7417 (1999); R. Rink, J. Kingma, J. H. L. Spelberg, and D. B. Janssen,
Biochemistry, 39, 5600 (2000).
239 S. Pedragosa-Moreau, A. Archelas, and R. Furstoss, J. Org. Chem., 58, 5533 (1993); S. Pedragosa-
Moreau, C. Morisseau, J. Zylber, A. Archelas, J. Baratti, and R. Furstoss, J. Org. Chem., 61, 7402
(1996); S. Pedragosa-Moreau, A. Archelas, and R. Furstoss, Tetrahedron, 52, 4593 (1996).
240
C. A. G. M. Weijers, Tetrahedron: Asymmetry, 8, 639 (1997); C. A. G. M. Weijers, A. L. Botes, M. S.
van Dyk, and J. A. M. de Bont, Tetrahedron: Asymmetry, 9, 467 (1998).