Page 249 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 249
in the molecule is bonded to an anomeric carbon and is subject to the anomeric 229
effect. Equally striking is the observation that all the substituents of the tri-O-acetyl-
ß-D-xylopyranosyl chloride shown in Entry 5 are in the axial orientation in solution. TOPIC 2.3
Here, no special crystal packing forces can favor the preferred conformation. The The Anomeric Effect in
Cyclic Compounds
anomeric effect of a single chlorine is sufficient to drive the equilibrium in favor of
the conformation that puts the three acetoxy groups in axial positions.
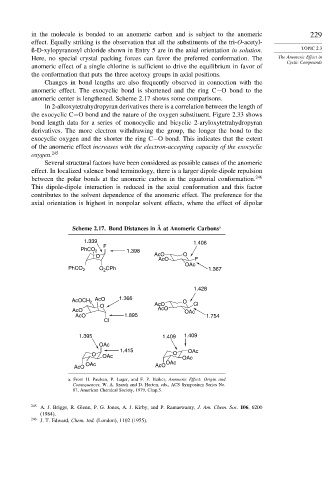
Changes in bond lengths are also frequently observed in connection with the
anomeric effect. The exocyclic bond is shortened and the ring C−O bond to the
anomeric center is lengthened. Scheme 2.17 shows some comparisons.
In 2-alkoxytetrahydropyran derivatives there is a correlation between the length of
the exocyclic C−O bond and the nature of the oxygen substituent. Figure 2.33 shows
bond length data for a series of monocyclic and bicyclic 2-aryloxytetrahydropyran
derivatives. The more electron withdrawing the group, the longer the bond to the
exocyclic oxygen and the shorter the ring C−O bond. This indicates that the extent
of the anomeric effect increases with the electron-accepting capacity of the exocyclic
oxygen. 245
Several structural factors have been considered as possible causes of the anomeric
effect. In localized valence bond terminology, there is a larger dipole-dipole repulsion
between the polar bonds at the anomeric carbon in the equatorial conformation. 246
This dipole-dipole interaction is reduced in the axial conformation and this factor
contributes to the solvent dependence of the anomeric effect. The preference for the
axial orientation is highest in nonpolar solvent effects, where the effect of dipolar
Scheme 2.17. Bond Distances in Å at Anomeric Carbons a
1.339 1.406
F
PhCO 2 1.398
O AcO O
AcO F
OAc
PhCO 2 O 2 CPh 1.367
1.428
AcOCH 2 AcO 1.366 O
O AcO Cl
AcO AcO OAc
AcO 1.895 1.754
Cl
1.395 1.409 1.409
OAc
1.415 OAc
O OAc O OAc
OAc OAc
AcO AcO
a. From H. Paulsen, P. Luger, and F. P. Heiker, Anomeric Effect: Origin and
Consequences, W. A. Szarek and D. Horton, eds., ACS Symposium Series No.
87, American Chemical Society, 1979, Chap.5.
245 A. J. Briggs, R. Glenn, P. G. Jones, A. J. Kirby, and P. Ramaswamy, J. Am. Chem. Soc. 106, 6200
(1984).
246
J. T. Edward, Chem. Ind. (London), 1102 (1955).