Page 250 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 250
230
CHAPTER 2
Stereochemistry,
Conformation,
and Stereoselectivity
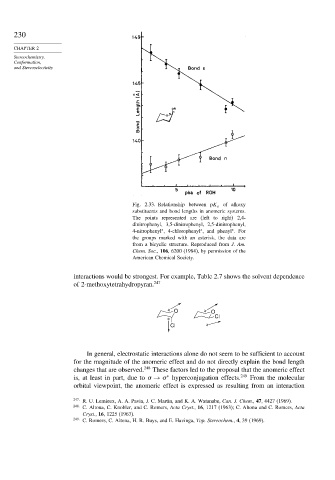
Fig. 2.33. Relationship between pK a of alkoxy
substituents and bond lengths in anomeric systems.
The points represented are (left to right) 2,4-
dinitrophenyl, 3,5-dinitrophenyl, 2,5-dinitrophenyl,
4-nitrophenyl , 4-chlorophenyl , and phenyl . For
∗
∗
∗
the groups marked with an asterisk, the data are
from a bicyclic structure. Reproduced from J. Am.
Chem. Soc., 106, 6200 (1984), by permission of the
American Chemical Society.
interactions would be strongest. For example, Table 2.7 shows the solvent dependence
of 2-methoxytetrahydropyran. 247
+ O + O
Cl
+
Cl +
In general, electrostatic interactions alone do not seem to be sufficient to account
for the magnitude of the anomeric effect and do not directly explain the bond length
changes that are observed. 248 These factors led to the proposal that the anomeric effect
∗
is, at least in part, due to → hyperconjugation effects. 249 From the molecular
orbital viewpoint, the anomeric effect is expressed as resulting from an interaction
247
R. U. Lemieux, A. A. Pavia, J. C. Martin, and K. A. Watanabe, Can. J. Chem., 47, 4427 (1969).
248 C. Altona, C. Knobler, and C. Romers, Acta Cryst., 16, 1217 (1963); C. Altona and C. Romers, Acta
Cryst., 16, 1225 (1963).
249
C. Romers, C. Altona, H. R. Buys, and E. Havinga, Top. Stereochem., 4, 39 (1969).