Page 255 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 255
235
TOPIC 2.4
Polar Substituent Effects
in Reduction of Carbonyl
Compounds
∗
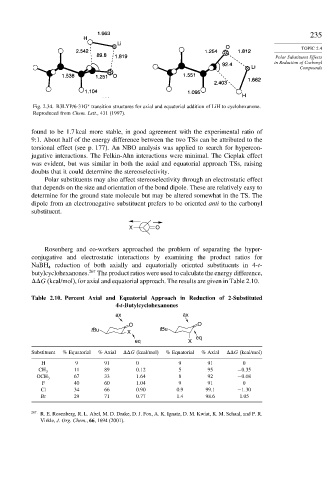
Fig. 2.34. B3LYP/6-31G transition structures for axial and equatorial addition of LiH to cyclohexanone.
Reproduced from Chem. Lett., 431 (1997).
found to be 1.7 kcal more stable, in good agreement with the experimental ratio of
9:1. About half of the energy difference between the two TSs can be attributed to the
torsional effect (see p. 177). An NBO analysis was applied to search for hypercon-
jugative interactions. The Felkin-Ahn interactions were minimal. The Cieplak effect
was evident, but was similar in both the axial and equatorial approach TSs, raising
doubts that it could determine the stereoselectivity.
Polar substituents may also affect stereoselectivity through an electrostatic effect
that depends on the size and orientation of the bond dipole. These are relatively easy to
determine for the ground state molecule but may be altered somewhat in the TS. The
dipole from an electronegative substituent prefers to be oriented anti to the carbonyl
substituent.
X O
Rosenberg and co-workers approached the problem of separating the hyper-
conjugative and electrostatic interactions by examining the product ratios for
NaBH 4 reduction of both axially and equatorially oriented substituents in 4-t-
butylcyclohexanones. 267 The product ratios were used to calculate the energy difference,
G (kcal/mol), for axial and equatorial approach. The results are given in Table 2.10.
Table 2.10. Percent Axial and Equatorial Approach in Reduction of 2-Substituted
4-t-Butylcyclohexanones
ax ax
O O
tBu tBu
X
eq
eq X
Substituent % Equatorial % Axial G (kcal/mol) % Equatorial % Axial G (kcal/mol)
H 9 91 0 9 91 0
11 89 0 12 5 95 −0 35
CH 3
67 33 1 64 8 92 −0 08
OCH 3
F 40 60 1 04 9 91 0
Cl 34 66 0 90 0.9 99.1 −1 30
Br 29 71 0 77 1.4 98.6 1.05
267
R. E. Rosenberg, R. L. Abel, M. D. Drake, D. J. Fox, A. K. Ignatz, D. M. Kwiat, K. M. Schaal, and P. R.
Virkle, J. Org. Chem., 66, 1694 (2001).