Page 256 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 256
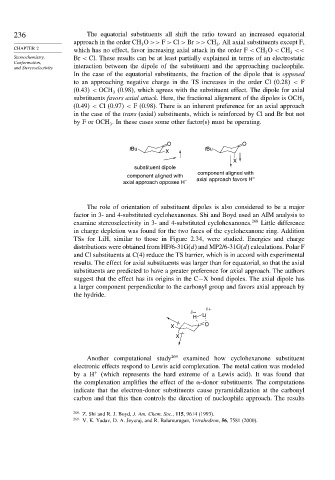
236 The equatorial substituents all shift the ratio toward an increased equatorial
approach in the order CH O >> F > Cl > Br >> CH . All axial substituents except F,
3 3
CHAPTER 2 which has no effect, favor increasing axial attack in the order F < CH O < CH <<
3 3
Stereochemistry, Br < Cl. These results can be at least partially explained in terms of an electrostatic
Conformation,
and Stereoselectivity interaction between the dipole of the substituent and the approaching nucleophile.
In the case of the equatorial substituents, the fraction of the dipole that is opposed
to an approaching negative charge in the TS increases in the order Cl (0.28) < F
(0.43) < OCH (0.98), which agrees with the substituent effect. The dipole for axial
3
substituents favors axial attack. Here, the fractional alignment of the dipoles is OCH 3
(0.49) < Cl (0.97) < F (0.98). There is an inherent preference for an axial approach
in the case of the trans (axial) substituents, which is reinforced by Cl and Br but not
byForOCH . In these cases some other factor(s) must be operating.
3
O O
tBu tBu
X
X
substituent dipole
component aligned with
component aligned with –
axial approach opposes H – axial approach favors H
The role of orientation of substituent dipoles is also considered to be a major
factor in 3- and 4-substituted cyclohexanones. Shi and Boyd used an AIM analysis to
examine stereoselectivity in 3- and 4-substituted cyclohexanones. 268 Little difference
in charge depletion was found for the two faces of the cyclohexanone ring. Addition
TSs for LiH, similar to those in Figure 2.34, were studied. Energies and charge
distributions were obtained from HF/6-31G(d) and MP2/6-31G(d) calculations. Polar F
and Cl substituents at C(4) reduce the TS barrier, which is in accord with experimental
results. The effect for axial substituents was larger than for equatorial, so that the axial
substituents are predicted to have a greater preference for axial approach. The authors
suggest that the effect has its origins in the C−X bond dipoles. The axial dipole has
a larger component perpendicular to the carbonyl group and favors axial approach by
the hydride.
δ +
δ −
H Li
X O
X
Another computational study 269 examined how cyclohexanone substituent
electronic effects respond to Lewis acid complexation. The metal cation was modeled
byaH + (which represents the hard extreme of a Lewis acid). It was found that
the complexation amplifies the effect of the -donor substituents. The computations
indicate that the electron-donor substituents cause pyramidalization at the carbonyl
carbon and that this then controls the direction of nucleophile approach. The results
268 Z. Shi and R. J. Boyd, J. Am. Chem. Soc., 115, 9614 (1993).
269
V. K. Yadav, D. A. Jeyaraj, and R. Balamurugan, Tetrahedron, 56, 7581 (2000).