Page 257 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 257
are summarized below. According to NPA charge analysis, the Cl and SH substituents 237
are significant donors and lead to movement of oxygen to the equatorial direction,
favoring axial approach by the nucleophile. The oxygen and fluoro substituents have TOPIC 2.4
the opposite effects and favor equatorial approach. Experimental data are available for Polar Substituent Effects
in Reduction of Carbonyl
the SH, Cl, and OCH substituents and are in accord with these predictions. Looking Compounds
3
at the data in Table 2.10, we see that axial OCH and F have little effect, whereas
3
Cl and Br favor axial approach. These results are in agreement with the better donor
capacity attributed to third-row elements in the discussion of heteroatom hyperconju-
gation (Topic 1.2). This study concludes that the substituents effects operate in the
ground state molecule and are accentuated by coordination of a cation at oxygen.
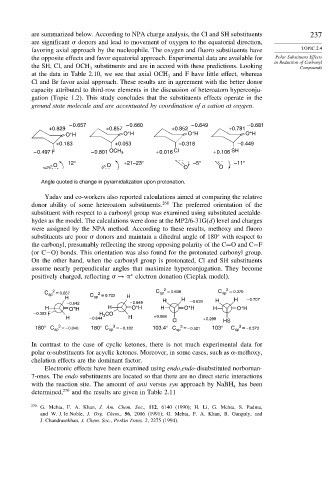
– 0.657 – 0.660 – 0.649 – 0.681
+0.829 +0.857 +0.852 +0.781
+
+
+
+
O H O H O H O H
+0.183 +0.053 –0.318 –0.449
– 0.497 F – 0.801 OCH 3 + 0.016 Cl + 0.106 SH
12° +21–23° –5° –11°
O O O O
Angle quoted is change in pyramidalization upon protonation.
Yadav and co-workers also reported calculations aimed at comparing the relative
donor ability of some heteroatom substituents. 268 The preferred orientation of the
substituent with respect to a carbonyl group was examined using substituted acetalde-
hydes as the model. The calculations were done at the MP2/6-31G(d) level and charges
were assigned by the NPA method. According to these results, methoxy and fluoro
substituents are poor donors and maintain a dihedral angle of 180 with respect to
the carbonyl, presumably reflecting the strong opposing polarity of the C=O and C−F
(or C−O) bonds. This orientation was also found for the protonated carbonyl group.
On the other hand, when the carbonyl group is protonated, Cl and SH substituents
assume nearly perpendicular angles that maximize hyperconjugation. They become
∗
positively charged, reflecting → electron donation (Cieplak model).
C sp 2 = 0.667 C 2 C sp 2 = 0.636 C sp 2 = 0.370
H sp = 0.702 H H H H H – 0.707
– 0.642 – 0.649 – 0.639
+
+
+
+
H O H H O H H O H H O H
– 0.383 F H 3 CO
H – 0.644 H + 0.068 + 0.299
Cl HS
180° C sp 3 = – 0.040 180° C sp 3 = – 0.182 103.4° C sp 3 = – 0.521 103° C sp 3 = – 0.573
In contrast to the case of cyclic ketones, there is not much experimental data for
polar -substituents for acyclic ketones. Moreover, in some cases, such as -methoxy,
chelation effects are the dominant factor.
Electronic effects have been examined using endo,endo-disubstituted norbornan-
7-ones. The endo substituents are located so that there are no direct steric interactions
with the reaction site. The amount of anti versus syn approach by NaBH has been
4
determined, 270 and the results are given in Table 2.11
270
G. Mehta, F. A. Khan, J. Am. Chem. Soc., 112, 6140 (1990); H. Li, G. Mehta, S. Padma,
and W. J. le Noble, J. Org. Chem., 56, 2006 (1991); G. Mehta, F. A. Khan, B. Ganguly, and
J. Chandrasekhan, J. Chem. Soc., Perkin Trans. 2, 2275 (1994).