Page 262 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 262
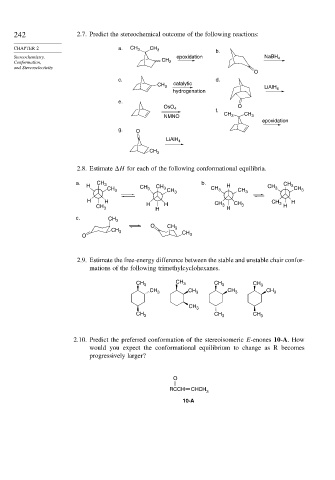
242 2.7. Predict the stereochemical outcome of the following reactions:
CHAPTER 2 a. CH 3 CH 3
b.
Stereochemistry, epoxidation NaBH 4
Conformation, CH 2
and Stereoselectivity
O
c. d.
catalytic
CH 3
LiAlH 4
hydrogenation
e.
O
OsO 4
f.
NMNO CH 3 CH 3
epoxidation
g. O
LiAlH 4
CH 3
2.8. Estimate H for each of the following conformational equilibria.
a. CH b. CH
H 3 CH CH H CH 3
CH 3 3 3 CH 3 CH 3 CH 3 3 CH 3
H H CH CH 3 H
CH 3 H H H 3 H CH 3 H
c. CH 3
O CH
CH 3 3
O CH 3
2.9. Estimate the free-energy difference between the stable and unstable chair confor-
mations of the following trimethylcyclohexanes.
CH 3 CH 3 CH 3 CH 3
CH 3 CH 3 CH 3 CH 3
CH 3
CH 3 CH 3 CH 3
2.10. Predict the preferred conformation of the stereoisomeric E-enones 10-A. How
would you expect the conformational equilibrium to change as R becomes
progressively larger?
O
RCCH CHCH
3
10-A