Page 265 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 265
245
PROBLEMS
1
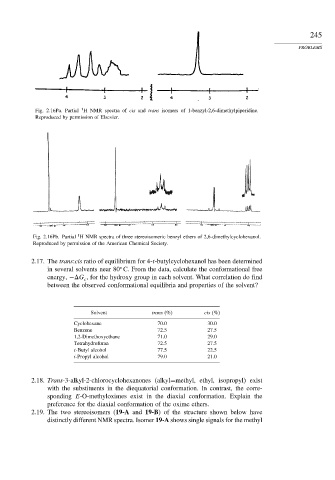
Fig. 2.16Pa. Partial H NMR spectra of cis and trans isomers of 1-benzyl-2,6-dimethylpiperidine.
Reproduced by permission of Elsevier.
1
Fig. 2.16Pb. Partial H NMR spectra of three stereoisomeric benzyl ethers of 2,6-dimethylcyclohexanol.
Reproduced by permission of the American Chemical Society.
2.17. The trans:cis ratio of equilibrium for 4-t-butylcyclohexanol has been determined
in several solvents near 80 C. From the data, calculate the conformational free
energy, − G , for the hydroxy group in each solvent. What correlation do find
c
between the observed conformational equilibria and properties of the solvent?
Solvent trans (%) cis (%)
Cyclohexane 70.0 30.0
Benzene 72.5 27.5
1,2-Dimethoxyethane 71.0 29.0
Tetrahydrofuran 72.5 27.5
t-Butyl alcohol 77.5 22.5
i-Propyl alcohol 79.0 21.0
2.18. Trans-3-alkyl-2-chlorocyclohexanones (alkyl=methyl, ethyl, isopropyl) exist
with the substituents in the diequatorial conformation. In contrast, the corre-
sponding E-O-methyloximes exist in the diaxial conformation. Explain the
preference for the diaxial conformation of the oxime ethers.
2.19. The two stereoisomers (19-A and 19-B) of the structure shown below have
distinctly different NMR spectra. Isomer 19-A shows single signals for the methyl