Page 270 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 270
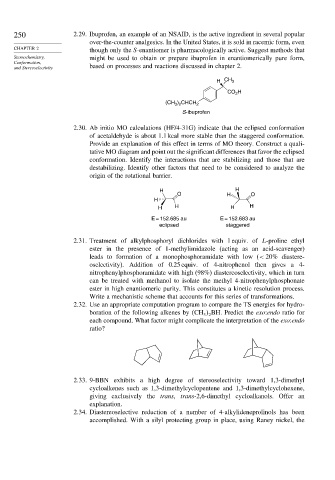
250 2.29. Ibuprofen, an example of an NSAID, is the active ingredient in several popular
over-the-counter analgesics. In the United States, it is sold in racemic form, even
CHAPTER 2 though only the S-enantiomer is pharmacologically active. Suggest methods that
Stereochemistry, might be used to obtain or prepare ibuprofen in enantiomerically pure form,
Conformation,
and Stereoselectivity based on processes and reactions discussed in chapter 2.
H CH 3
CO H
2
(CH ) CHCH 2
3 2
S-ibuprofen
2.30. Ab initio MO calculations (HF/4-31G) indicate that the eclipsed conformation
of acetaldehyde is about 1.1 kcal more stable than the staggered conformation.
Provide an explanation of this effect in terms of MO theory. Construct a quali-
tative MO diagram and point out the significant differences that favor the eclipsed
conformation. Identify the interactions that are stabilizing and those that are
destabilizing. Identify other factors that need to be considered to analyze the
origin of the rotational barrier.
H H
O H O
H
H H H H
E = 152.685 au E = 152.683 au
eclipsed staggered
2.31. Treatment of alkylphosphoryl dichlorides with 1 equiv. of L-proline ethyl
ester in the presence of 1-methylimidazole (acting as an acid-scavenger)
leads to formation of a monophosphoramidate with low (< 20% diastere-
oselectivity). Addition of 0.25 equiv. of 4-nitrophenol then gives a 4-
nitrophenylphosphoramidate with high (98%) diastereoselectivity, which in turn
can be treated with methanol to isolate the methyl 4-nitrophenylphosphonate
ester in high enantiomeric purity. This constitutes a kinetic resolution process.
Write a mechanistic scheme that accounts for this series of transformations.
2.32. Use an appropriate computation program to compare the TS energies for hydro-
boration of the following alkenes by CH BH. Predict the exo:endo ratio for
3 2
each compound. What factor might complicate the interpretation of the exo:endo
ratio?
2.33. 9-BBN exhibits a high degree of stereoselectivity toward 1,3-dimethyl
cycloalkenes such as 1,3-dimethylcyclopentene and 1,3-dimethylcyclohexene,
giving exclusively the trans, trans-2,6-dimethyl cycloalkanols. Offer an
explanation.
2.34. Diastereoselective reduction of a number of 4-alkylideneprolinols has been
accomplished. With a silyl protecting group in place, using Raney nickel, the