Page 266 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 266
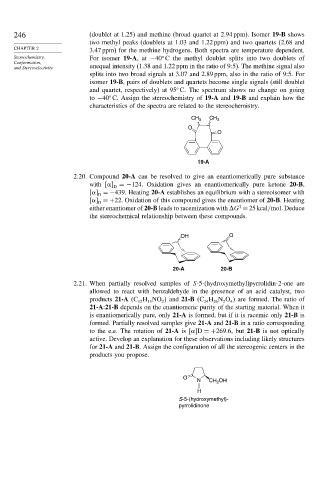
246 (doublet at 1.25) and methine (broad quartet at 2.94 ppm). Isomer 19-B shows
two methyl peaks (doublets at 1.03 and 1.22 ppm) and two quartets (2.68 and
CHAPTER 2 3.47 ppm) for the methine hydrogens. Both spectra are temperature dependent.
Stereochemistry, For isomer 19-A,at −40 C the methyl doublet splits into two doublets of
Conformation,
and Stereoselectivity unequal intensity (1.38 and 1.22 ppm in the ratio of 9:5). The methine signal also
splits into two broad signals at 3.07 and 2.89 ppm, also in the ratio of 9:5. For
isomer 19-B, pairs of doublets and quartets become single signals (still doublet
and quartet, respectively) at 95 C. The spectrum shows no change on going
to −40 C. Assign the stereochemistry of 19-A and 19-B and explain how the
characteristics of the spectra are related to the stereochemistry.
CH 3 CH 3
O
O
19-A
2.20. Compound 20-A can be resolved to give an enantiomerically pure substance
with =−124. Oxidation gives an enantiomerically pure ketone 20-B,
D
=−439. Heating 20-A establishes an equilibrium with a stereoisomer with
D
=+22. Oxidation of this compound gives the enantiomer of 20-B. Heating
D
‡
either enantiomer of 20-B leads to racemization with G = 25kcal/mol. Deduce
the stereochemical relationship between these compounds.
OH O
20-A 20-B
2.21. When partially resolved samples of S-5-(hydroxymethyl)pyrrolidin-2-one are
allowed to react with benzaldehyde in the presence of an acid catalyst, two
products 21-A C H NO and 21-B C H N O are formed. The ratio of
24
4
2
12
13
26
2
21-A:21-B depends on the enantiomeric purity of the starting material. When it
is enantiomerically pure, only 21-A is formed, but if it is racemic only 21-B is
formed. Partially resolved samples give 21-A and 21-B in a ratio corresponding
to the e.e. The rotation of 21-A is D =+269 6, but 21-B is not optically
active. Develop an explanation for these observations including likely structures
for 21-A and 21-B. Assign the configuration of all the stereogenic centers in the
products you propose.
O
N CH OH
2
H
S-5-(hydroxymethyl)-
pyrrolidinone