Page 268 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 268
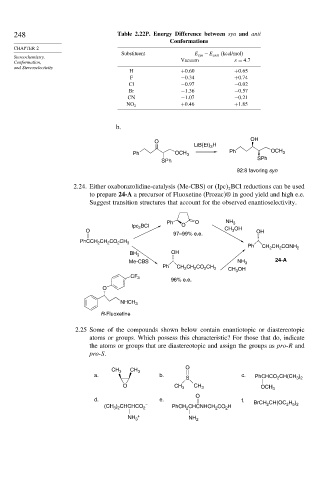
248 Table 2.22P. Energy Difference between syn and anti
Conformations
CHAPTER 2
Substituent E syn −E anti (kcal/mol)
Stereochemistry,
Conformation, Vacuum = 4 7
and Stereoselectivity
H +0 60 +0 65
F −0 34 +0 74
Cl −0 97 −0 02
Br −1 36 −0 57
CN −1 07 −0 21
+0 46 +1 85
NO 2
b.
OH
O
LiB(Et) H
3
Ph OCH 3 Ph OCH 3
SPh
SPh
92:8 favoring syn
2.24. Either oxaborazolidine-catalysis (Me-CBS) or Ipc BCl reductions can be used
2
to prepare 24-A a precursor of Fluoxetine (Prozac)® in good yield and high e.e.
Suggest transition structures that account for the observed enantioselectivity.
Ph O NH 3
Ipc 2 BCl O
O CH 3 OH OH
97–99% e.e.
PhCCH 2 CH 2 CO 2 CH 3
Ph CH 2 CH 2 CONH 2
OH
BH 3
Me-CBS NH 3 24-A
Ph
CH 2 CH 2 CO 2 CH 3
CH 3 OH
CF 3
96% e.e.
O
NHCH 3
R-Fluoxetine
2.25 Some of the compounds shown below contain enantiotopic or diastereotopic
atoms or groups. Which possess this characteristic? For those that do, indicate
the atoms or groups that are diastereotopic and assign the groups as pro-R and
pro-S.
O
CH 3 CH 3
a. b. c. CH(CH )
S PhCHCO 2 3 2
O CH 3 CH 3 OCH 3
O
d. e. f.
(CH ) CHCHCO 2 – PhCH CHCNHCH CO H BrCH 2 CH(OC 2 H 5 ) 2
2
3 2
2
2
NH 3 + NH 2